Introduction: Allogeneic stem cell transplant (HCT) is the only potential curative option for patients with inherited marrow failure (iBMF) and myeloid germline predisposition syndromes (GPD). HCT outcomes are influenced by inherent disease specific-nuances such as alkylating agent and radio-sensitivity, immune deregulation, and higher risks for graft failure and GVHD; factors contributing to transplant related mortality (TRM) and morbidity. We carried out this retrospective study to assess survival outcomes and long term complications (LTC) in patients with IBMF and GPD that underwent HCT.
Patients and methods: We queried our institutional database and identified patients with iBMF and GPD as defined by the 2016 WHO classification. These included Fanconi anemia (FA), short telomere syndromes (STS), Diamond-Blackfan anemia (DBA), GATA2 and RUNX1 haploinsufficiency, congenital amegakaryocytic thrombocytopenia (CAMT), deficiency of adenosine deaminase 2 (DADA2), among others. Patients with acquired causes of BMF were excluded. Statistical analyses were performed using SAS (JMP v14.1).
Results: Twenty eight patients, median age 10 years (1 month-63 years), 46% males, were included in the study (table 1).
Fanconi anemia
Seven (25%) patients with FA underwent HCT, 5 (71%) without myeloid transformation and 2 after transformation to MDS/AML. Five (71%) patients received a RIC, 4 (57%) prior to transformation. At a median follow up (FU) of 126 m, the median OS was 194 m (95% CI 34m; NR) and 10 year survival was 64%. Grade 1 aGVHD was seen in four (57%) and 3 (42%) developed mild cGVHD, while 1 developed a donor derived AML (sibling not tested for FA). LTC included second primary malignancy (SPM) - squamous cell cancer (SCC) of skin and muscle invasive bladder cancer (MIBC) in 1(14%) and SCC of head/neck and anogenital region in 3 patients (43%), psychosocial complications (PS) in 6(85%), premature ovarian failure (POF) in 3(43%), and avascular necrosis (AVN) in 4(57%) patients.
Short Telomere Syndromes (STS)
Seven patients with STS underwent HCT, 2 (28%) after transformation to MDS. Five (71%) received RIC, including both the transformed patients. At a median FU of 67m, median OS was NR (95% CI 2m; NR) and 5 year survival was 47%. One (14%) patient developed grade 2 aGVHD and mild cGVHD of skin. Three (43%) developed SPM - skin cancers in 2 and MIBC in 1. PS was noted in 1(15%), and AVN in 3(43%). Three (43%) patients had concomitant mild IPF/restrictive lung disease.
GATA2 haploinsufficiency:
Seven patients with GATA2 haploinsufficiency underwent HCT; 2(28.5%) after transformation to MDS and 3(43%) to AML, of which 2(40%) received MAC. At a median FU of 57m, median OS was NR (95% CI 7m; NR) and 5 year survival was 71%. Three (50%) developed grade 2 aGVHD of skin and GI tract and 2(33%) developed mild cGVHD. LTC include PTLD, AVN and POF in 1 patient each.
Ribosomopathies:
One patient (13y) with DBA underwent RIC HCT and developed grade 2 aGVHD, secondary iron overload, and died at 10 months due to severe fungal infection.
Others:
Identical twins with CAMT underwent HCT (at 4y and 5y) from the same unrelated donor and at last FU (74m and 87m, respectively) remain 100% donor without GVHD. Two children with primary immunodeficiency and marrow failure underwent HCT (at 2y and 7y), one after transformation to MDS. The non-transformed patient is currently alive (120m), while the patient with transformation died 1month after HCT from disseminated cytomegalovirus infection. One patient with germline RUNX1 deletion developed CMML and underwent a RIC HCT and is alive at a FU of 4 months, with no GVHD. One patient with DADA2 (n=1) underwent RIC HCT without LTC.
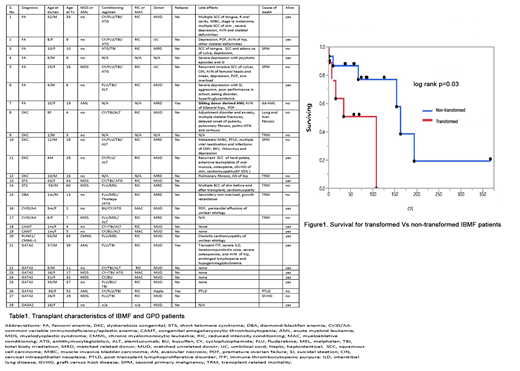
Due to the smaller cohort size, we compared OS in transformed and non-transformed patients for the IBMF and GATA2 patients only (n=24, 9 with transformation) (figure1). At a median FU of 74m, the median OS of transformed vs non-transformed patients was 108m(95% CI 1;108m) and 163m(95% CI 67m; NR), respectively (p=0.033).
Conclusions: Our study demonstrates that HCT remains an important intervention for IBMF and GPD, with the maximum impact being gained prior to transformation. While only mild chronic GVHD was noted (37%), inherent syndromic issues resulted in a high rate of SPM (FA/STS) and organ failure (STS- IPF). Notably, one FA patient who received an MRD HCT developed donor derived AML, underlining the importance of genetic screening in asymptomatic related individuals.
Kenderian:Lentigen: Research Funding; Kite/Gilead: Research Funding; Morphosys: Research Funding; Humanigen: Other: Scientific advisory board , Patents & Royalties, Research Funding; Novartis: Patents & Royalties, Research Funding; Tolero: Research Funding. Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal