Introduction: Hematopoietic stem cell (HSC) gene therapy/editing is a viable treatment option for various hematological diseases and disorders including hemoglobinopathies and HIV/AIDS. Most if not all currently available approaches target CD34-enriched cell fractions, a heterogeneous mix of mostly committed progenitor cells and only very few true HSCs with long-term multilineage engraftment potential. As a consequence, gene therapy/editing approaches are currently limited in their HSC targeting efficiency, very expensive consuming huge quantities of modifying reagents, and can lead to unwanted side-effects in non-target cells.
We recently described a novel HSC-enriched CD34 subset (CD90+CD45RA-) that is exclusively responsible for rapid recovery onset, robust long-term multilineage engraftment, as well as entire reconstitution of the bone marrow stem cell compartment in the nonhuman primate (NHP) stem cell transplantation and gene therapy model (Radtke et al. 2017, STM). Most importantly, we demonstrate that this CD34 subset reduces the number of target cells, modifying reagents and costs by more than 10-fold without compromising the long-term efficiency of gene-modification in the NHP (Humbert and Radtke et al. 2019, STM). Here, we aimed to develop a clinical protocol to reliably purify and efficiently gene-modify human HSC-enriched CD90+ cell fractions.
Methods: Large-scale enrichment of CD34+ cells from GCSF-mobilized leukapheresis products was initially performed on the Miltenyi CliniMACS Prodigy according to previously established protocols (Adair et al. 2017, Nat. Comm.). Yield, purity, quality, and feasibility of CD90 sorting was then comprehensively tested on two different commercially available cell sorting systems comparing the jet-in-air sorter FX500 from Sony and the cartridge-based closed-system sorter MACSQuant Tyto from Miltenyi Biotech with our clinically approved gold-standard CD34-mediated gene therapy approach. Sorted CD90+ and bulk CD34+ cells were transduced with a clinical-grade lentivirus encoding for GFP and the multilineage differentiation as well as engraftment potential tested using in vitro assays and the NSG mouse xenograft model, respectively.
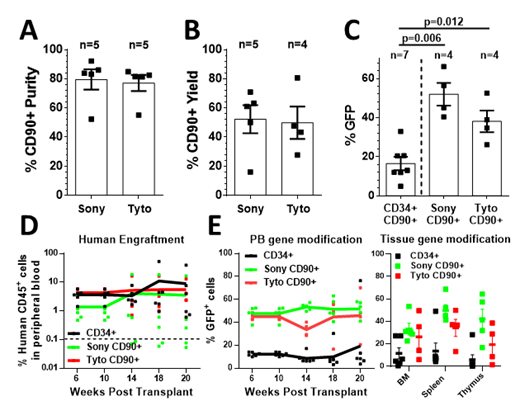
Results: Flow-cytometric sort-purification of CD90+ cells was similarly efficient in purity and yield using either the FX500 or Tyto (Figure A,B). Both approaches reliably reduced the overall target cell count by 10 to 15-fold without impacting the cells viability and in vitro colony-forming cell potential. Unexpectedly, the transduction efficiency of sort-purified CD90+ cells was significantly improved compared to bulk-transduced CD34+ cells and especially the CD34+CD90+ subset (Figure C). All cell fractions demonstrated robust mouse xenograft potential (Figure D). Most importantly, significantly higher levels of GFP+ expression in the peripheral blood, bone marrow, spleen and thymus were observed after transplantation of gene-modified CD90+ compared to bulk CD34+ cells in NSG mice (Figure E).
Conclusion: Here, we show that sort-purification of our HSC-enriched CD34+CD90+ cell subset is technically feasible and highly reproducible in two different systems. Purification of human CD90+ cell fractions significantly increased the gene-modification efficiency of primitive human HSCs with multilineage mouse engraftment potential. These findings should have important implications for currently available as well as future HSC gene therapy and gene editing protocols. Isolation of an HSC-enriched phenotype will allow more targeted gene modification and thus likely reduce unwanted off target effects. Our approach further reduced the overall costs for gene modifying reagents, can be combined with a closed transduction system, increase the portability and ultimately make HSC gene therapy GMP-facility independent and affordable. Finally, this stem cell selection strategy may also allow efficient and effective depletion of donor T cells in the setting of allogeneic stem cell or organ transplantation.
Figure: A) Purity and B) yield of CD90+ cells after sort-purification. C) Transduction efficiency of bulk-transduced CD34+CD90+ cells and sort-purified CD90+ cells. Frequency of D) human chimerism and E) GFP+ human CD45+ cells in the peripheral blood (PB), bone marrow, spleen and thymus after transplantation of gene-modified bulk CD34+ or sort-purified CD90+ cells.
Kiem:CSL Behring: Consultancy; Rocket Pharma: Consultancy, Equity Ownership; Homology Medicines: Consultancy, Equity Ownership; Magenta Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal