BACKGROUND: KappaMab is a chimeric IgG1 monoclonal antibody specific for Kappa Myeloma antigen (KMA), a tumour specific cell antigen exclusively expressed on the surface of kappa restricted MM cells. Early safety and efficacy signals seen with single-agent treatment in phase I/II studies in conjunction with observations that IMiD®-treatment upregulates the KMA target and enhances effector cell cytotoxicity, provide rationale for this proof-of principal immune-oncology (IO) approach in a kappa restricted MM population.
AIMS: To establish the clinical benefit rate (CBR) of KappaMab alone (Stage 1) and in combination with lenalidomide (LEN) and low dose dexamethasone (DEX) (Stage 2). Secondary aims: to determine the safety of KappaMab in combination with LEN and DEX, in particular, the incidence of immunological adverse events (AEs); to evaluate kinetics of response (time to response [TTOR], PFS, OS).
METHODS: Investigator initiated, phase IIb, multi-centre, open label sequential cohort study comparing KappaMab alone to KappaMab in combination with LEN and DEX in relapsed/refractory (RR) MM. Key inclusion criteria were kappa-restricted MM, 1-3 prior lines of therapy but no prior LEN. Recruitment was planned for 60 patients, with an initial intention to treat 30 patients per stage. In Stage 1, patients received KappaMab (10mg/kg IV infusion) weekly for 8/52 (induction), then every 4/52 (maintenance). [One cycle = 28d] For Stage 2, KappaMab dosing was as per stage 1 with the addition of LEN (25mg D1-21) and DEX 40mg weekly. In cycle 1 of Stage 2, LEN and DEX commenced 1/52 prior to KappaMab. [Cycle 1 = 35d: LEN 25mg D1-28 and DEX 40mg weekly (D1, 8, 15, 22, 29)]. Treatment continued until toxicity/progression. This is a planned interim analysis of the primary endpoint (CBR).
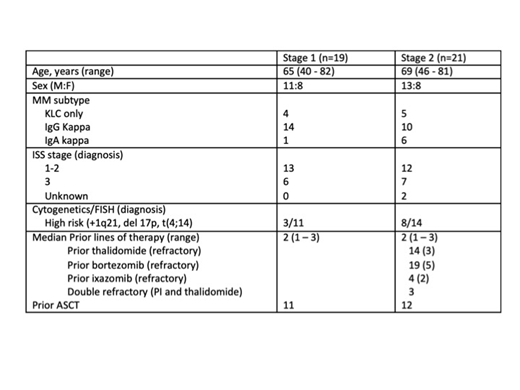
RESULTS: Recruitment has completed (n=59), however only 40 patients are included in this analysis (Table 1), enrolled between November 2016 and January 2019. Following review by the DMC, recruitment to Stage 1 was terminated early (n=19). 40 patients have commenced treatment in Stage 2, however only the first 21 are included in this analysis: 14/21 had prior thalidomide (refractory =3), 19/21 had prior proteasome inhibitor (PI) (bortezomib = 19, ixazomib = 5; PI refractory = 7), 3/21 were double refractory. 12/40 patients remain on study (Stage 1=1, Stage 2=11). 20 patients have progressed (Stage 1=14, Stage 2=6), 6 withdrew consent (3 each stage), 1 other and 5 have died (Stage 1=2, Stage 2=3).
Observed CBR in Stage 1 was 5.3% (1/19, PR=1) compared to 76.6% in Stage 2 (16/21, VGPR=2, PR=12, MR=2). Proof of concept (PoC) criteria were not met for Stage 1, but were met for Stage 2: observed CBR ≥55%, and the posterior probability that the true CBR exceeds 35% was >0.95 (PP=0.999). Observed overall response rate (ORR) for Stage 1 was 5.3% (1/19, PR=1) compared to 66.7% (14/21) for Stage 2. Median TTOR was not reached in Stage 1 (95% CI: 4.6m and above), and was 2.0m in Stage 2 (95% CI: 1.2 - 2.3m) (p<0.001).
With an estimated median potential follow-up of 3.68m in Stage 1, 4.86m in stage 2, the median PFS for Stage 1 was 3.71m (95% CI: 1.68 - 5.52m), compared to 6.21m for Stage 2 (95% CI 3.88 - 11.0m) (p=0.010). Median OS for both stages was not reached: 95% CI 4.0m and above for Stage 1, and 4.9m and above for Stage 2 (p=0.500).
AEs of interest: Infusion reactions: 3/19 patients in Stage 1 (grade 1=1, grade 2=2), compared to 4/21 patients in Stage 2 (grade 2=4). Hematologic toxicities: There were no hematologic toxicities reported in Stage 1 in comparison to anaemia 3/21, neutropenia 5/21 (grade 3=3), thrombocytopenia 4/21 (grade 3=1, grade 4=2) reported in Stage 2. Non-hematologic AEs (regardless of causality and >10% incidence): musculoskeletal pain (Stage 1=5, Stage 2=6), fatigue (Stage 1=4, Stage 2=4), infection: URTI (Stage 1=5, Stage 2=4); LRTI (Stage 1=2, Stage 2=2), abdominal pain (stage 2=4), muscle cramps (stage 2=3), presyncope (Stage 2=3) and hypophosphatemia (Stage 2=3).
CONCLUSION: In a patient population with high prior IMiD (thalidomide) exposure and a median of 2 prior lines of therapy, KappaMab combined with LEN and DEX demonstrated a higher than expected ORR of 67%, comparing favourably with the MM-009/MM-010 trials of LEN and DEX, that demonstrated an ORR in patients with 1 prior line of 66.9%1. This novel IO combination may represent a promising new therapeutic option. This trial is ongoing.
1. E Stadtmauer et al European Journal of Haematology 2009; 82:426-432
Kalff:pfizer: Honoraria; Amgen: Honoraria; Celgene: Honoraria. Shortt:Takeda: Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Astex: Research Funding; Amgen: Research Funding; Gilead: Speakers Bureau. Reynolds:Alfred Health: Employment, Other: Biostatistician for trials funded by the Australian government and Abbvie, Amgen, Celgene, GSK, Janssen-Cilag, Merck, Novartis, Takeda, but sponsored by Alfred Health.; AUSTRALASIAN LEUKAEMIA & LYMPHOMA GROUP (ALLG): Consultancy; Novartis AG: Equity Ownership; Novartis Australia: Honoraria. Quach:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Walker:Peninsula Health: Employment; Alfred Health: Employment. Harrison:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dunn:Haemalogix: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Spencer:Amgen: Other: Consulting/advisory role, Research Funding; AbbVie: Other: Consulting/advisory role, Research Funding; Secura Bio: Other: Consulting/advisory role; Haemalogix: Other: Consulting/advisory role; Celgene: Other: Consulting/advisory role, Research Funding, Speakers Bureau; Sanofi: Other: Consulting/advisory role; Specialised Therapeutics Australia: Consultancy, Honoraria; Servier: Other: Consulting/advisory role; Janssen Oncology: Other: Consulting/advisory role, Research Funding, Speakers Bureau; Takeda: Other: Consulting/advisory role, Research Funding.
KappaMab - monoclonal AB directed against Kappa myeloma antigen
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal