Background
Recently, treatment options for RRMM have increased substantially with multiple approvals of novel agents/combination, making the treatment algorithm increasingly complex, with changes driven chiefly by access to novel agents/regimens. Furthermore, patient (pt) and disease characteristics have a profound impact on treatment decisions. To understand the impact of recently approved novel regimens on real-world (RW) treatment patterns, we conducted a multi-national survey to investigate the management of RRMM across Europe.
Methods
Retrospective, anonymized data from RRMM pts, treated in academic or community hospitals/clinics in 8 countries were extracted from Jan 2016 to Dec 2018. Data were analyzed overall and for Germany, Austria, and Switzerland (DACH) vs other countries (Belgium, France, Greece, Spain and UK) due to differences in treatment access.
Results
The cumulative number of pts included was 2782 in 2016, 3902 in 2017, and 4658 in 2018. Of the pts enrolled in 2016, 2017 and 2018, 40%, 49% and 51%, respectively, were in 3rd+ line (≥3L), potentially reflecting the increasing availability of treatment options for RRMM and extended survival in MM. Median age at diagnosis in pts enrolling in 2016, 2017, and 2018, was 68, 69, and 70 years, respectively, with 23%, 24%, 26% aged >75 years, underlining the fact that MM remains a disease of the elderly. The data revealed a difficult-to-treat RW population: 31%-36% of pts had an ECOG PS ≥2 at 2nd line (2L) in 2016-2018; increasing to 44%-49% at 4th+ lines (≥4L). At 2L, 42%-45% of pts presented ≥1 treatment-dependent comorbidity in 2016-2018, including hypertension (23-27%) and renal impairment (9-10%). Cytogenetic risk, evaluated in 38%-42% of pts at initial diagnosis, was reported as high in 8%-10% of the total population. Treatment initiation due to biochemical relapse was reported in 33%/36% of pts at 2L/3L in 2016, and in 30%/28% in 2018, indicating that ~1/3 of pts manifested an asymptomatic rather than clinical relapse.
The proportion of pts treated with triplet regimens increased from 26%, 26%, and 30% at 2L, 3L and ≥4L in 2016 to 43%, 40%, and 38% in 2018, reflecting the adoption of newly approved triplets in RRMM, particularly in DACH countries. Use of proteasome inhibitor (PI)-based regimens increased from 35%, 30% and 34% at 2L, 3L and ≥4L in 2016, to 43%, 37% and 37% in 2018, driven by increased/earlier use of novel PIs (carfilzomib and ixazomib). These trends were more obvious in DACH, highlighting the impact of earlier access to modern treatment in these countries. Similarly, the proportion of pts on daratumumab-based regimens increased from 0, 5%, and 20% at 2L, 3L and ≥4L in 2016, to 10%, 24% and 31% in 2018.
From 2016 to 2018, prior IMiD exposure at 2L increased from 11% to 20% in DACH, but remained stable at 42% in other countries; at 3L, there was an increase from 77% to 82% in all countries reflecting the uptake of novel triplet combinations. Most pts were IMiD-exposed or IMiD-refractory at ≥4L. Regarding the treatment algorithm, the rate of PI-based treatment at 1L was 74%-75%. PI- to IMiD-based therapy was the commonest treatment sequence from 1L to 2L, at 64%-66%, while PI- to PI-based therapy at 1L to 2L increased from 22% in 2016 to 30% in 2018.
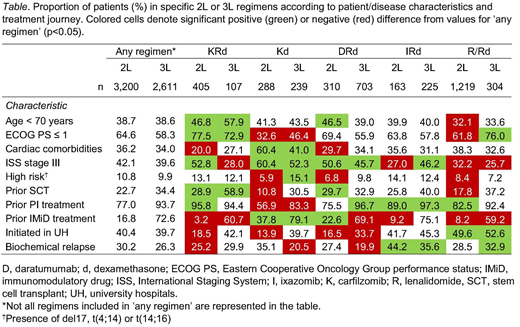
Key disease/pt characteristics associated with the selection of regimens at 2L and 3L are summarized in the Table. Prior IMiD treatment limited the use of IMiD-based therapy in subsequent lines. The use of KRd, IRd and DRd was mostly associated with ISS stage III, while the use of KRd was less frequently reported in pts with cardiac comorbidities. In pts with prior PI treatment, KRd and IRd (but not Kd) were more common at 2L, while DRd was preferred at 3L. A higher proportion of fit, young, or prior-SCT pts were treated with KRd or DRd, while IRd was the preferred treatment in pts with biochemical relapse.
Conclusions
Multiple drug approvals for RRMM in Europe have resulted in marked changes in the treatment algorithm, with a more immediate impact in countries with earlier access to new treatment options. Multiple decision drivers such as age, fitness, comorbidities and prior treatment are associated with uptake of different novel regimens at 2L and 3L. The increasing range of treatment options has resulted in pts receiving more lines of therapy for RRMM, highlighting the need for cautious planning of treatment sequencing to optimize the use of available combinations according to pt characteristics and disease factors.
Merz:Janssen: Other: Travel grants; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Abbvie: Other: Travel grants; Celgene: Other: Travel grants; Takeda Vertrieb GmbH: Other: Travel grants, Research Funding. Pérez:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees. Kolb:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: travel and registration for my participation to international medical congres (ASH). Symeonidis:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Tekeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding. Zomas:Takeda: Employment. Gonzalez:Takeda: Employment. Kellermann:Amgen: Research Funding; BMS: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Sanofi: Research Funding; Takeda: Research Funding. Goldschmidt:Chugai: Honoraria, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; John Hopkins University: Other: Grants and/or provision of Investigational Medicinal Product; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; ArtTempi: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; MSD: Research Funding; Molecular Partners: Research Funding; Mundipharma: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Dietmar-Hopp-Foundation: Other: Grants and/or provision of Investigational Medicinal Product.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal