Introduction: Regulatory T cells (Tregs) are potent immunosuppressors and are being developed as adoptive immunotherapy for inflammatory disorders and autoimmune diseases. Specifically, cord blood (CB) Tregs are emerging as a promising treatment for graft vs. host disease (GVHD) as well as immune mediated bone marrow failure disorders including aplastic anemia, hypoplastic myelodysplasia and primary myelofibrosis. We and others have shown that when compared to peripheral blood (PB), CB Tregs have a higher expression of Helios, a marker of thymic Tregs; exert superior suppression on proliferating conventional T cells and do not secrete inflammatory IL-17 or express RORүt under stressful conditions. Such a stable suppressor profile coupled with their ready availability as an off-the-shelf product positions CB Tregs to be a very attractive therapeutic agent. Since multiple myeloma, a plasma cell clonal malignancy, exists in an inflammatory microenvironment of hematopoietic and non-hematopoietic cells which is likely related to disease progression, we hypothesized that adoptive therapy with CB Tregs may block myeloma progression by exerting their anti-inflammatory function.
Methods: We used the NOD-SCID IL2rγnull (NSG) mice to first establish the myeloma tumor model, where mice were injected intravenously via tail vein with 3 x 106 Firefly luciferase labeled MM1S cells. For generating CB Tregs for adoptive therapy, Treg cell enrichment was performed from the whole CB unit using CD25+ magnetic beads and LS column (MIltenyl) followed by continuous culture in the presence of CD3/28 T-cell activator beads, and human recombinant interleukin-2 for 14 days. Expanded CB Treg phenotype was confirmed using flow cytometry analysis and the in-vitro suppressive capacity was assessed by CellTrace Violet Cell Proliferation assay. The mice were injected intravenously via tail vein with Firefly luciferase labeled MM1S cells on day 0 with or without 10 x 106 ex-vivo expanded CB Treg cells on day -1. We monitored the progression of MM1S cells by in vivo bioluminescence imaging (BLI) using the IVIS imaging system (Xenogen) 10 minutes after intraperitoneal injection of D-luciferin. Imaging data were analyzed and quantified with Living Image Software (Xenogen). We also assessed the weight and survival as well as the plasma cytokines levels. At the time of euthanasia, organs were harvested and analyzed for myeloma burden by using immunohistochemistry as well as flow analysis.
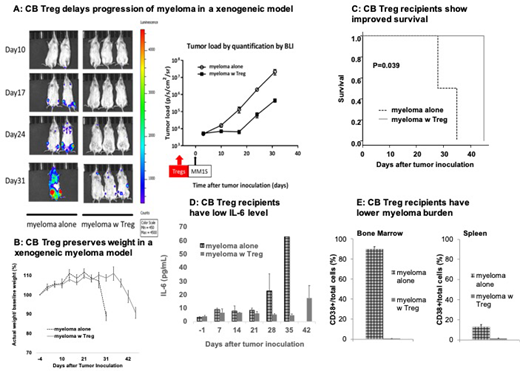
Results: The expanded CB Tregs showed a consistent phenotype of CD4+25+127-FoxP3high and more than 80% suppression of the proliferating conventional T cells (Tcons). The progression of MM1S cells in the mice injected with CB Treg (n=9) was significantly delayed as shown in figure 1A, where minimal evidence of myeloma cells is visualized on day 31 compared to widespread tumor in the control arm (n=9). CB Treg recipients preserved their weight for a longer time (Figure 1B) and had improved overall survival (p=0.039) (Figure 1C). The prevention of development of myeloma by CB Tregs correlated with the lower levels of the inflammatory cytokine, interleukin-6 (IL-6) (murine) especially on day 35 post myeloma inoculation (Figure 1D). The circulating myeloma cells in the CB Treg recipients were significantly lower as compared to control mice. Upon euthanasia on day 25 post inoculation, myeloma cells were barely detectable in bone marrow and spleen in the CB Treg recipients as compared to large tumor burden in the control arm (Figure 1E).
Conclusions: Our results demonstrate that ex-vivo expanded CB Treg cells prevent the growth of myeloma cells in a xenogenic model via suppression of inflammatory cytokines such as IL-6. These data provide additional insight to the underpinnings of myeloma progression and may contribute to the development of the novel immunotherapies in the future.
Shah:University of California, San Francisco: Employment; Genentech, Seattle Genetics, Oncopeptides, Karoypharm, Surface Oncology, Precision biosciences GSK, Nektar, Amgen, Indapta Therapeutics, Sanofi: Membership on an entity's Board of Directors or advisory committees; Indapta Therapeutics: Equity Ownership; Celgene, Janssen, Bluebird Bio, Sutro Biopharma: Research Funding; Poseida: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Nkarta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; Teneobio: Consultancy, Membership on an entity's Board of Directors or advisory committees. Orlowski:Poseida Therapeutics, Inc.: Research Funding. Parmar:Cellenkos Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal