Induced pluripotent stem cell (iPSC)-derived effector cells offer distinct advantages for immune therapy over existing patient- or donor- derived platforms, both in terms of scalable manufacturing from a renewable starting cellular material and precision genetic engineering that is performed at the single-cell level. iPSC derived natural killer (iNK) cells offer the further advantage of innate reactivity to stress ligands and MHC downregulation and the potential to recruit downstream adaptive responses. These unique features form the basis of our multi-antigen targeted chimeric antigen receptor (CAR) CAR-iNK cell product candidate, termed FT596, which is further combined with additional functionality to enhance effector function. FT596 is consistently manufactured from a master iPSC line engineered to uniformly express an NK cell-calibrated CD19-targeting CAR (CD19-CAR), an enhanced functioning high-affinity, non-cleavable CD16 (hnCD16) and a recombinant fusion of IL-15 and IL-15 receptor alpha (IL-15RF) for cytokine-autonomous persistence.
The design of the CD19-CAR involved exploiting the intrinsic polyfunctionality of NK cells, which function by engaging multiple signaling pathways activated through combinations of distinct germline encoded receptors. Using this approach, the transmembrane region of activating receptor NKG2D, combined with the intracellular signaling domains of SLAM co-receptor 2B4 and CD3ζ, proved the most effective in triggering antigen specific functional responses in NK cells. Chimerization of an anti-CD19 scFv onto this NKG2D-2B4-CD3ζ signaling platform produced specific in vitro recognition of CD19+ B cell lymphoma cells in short-term and long-term NK cytotoxicity assays (>80% and <40% clearance of tumor cells at 60H, p<0.001 respectively).
The functionality of the CD19-CAR was further enhanced in combination with autonomous IL-15 signaling. Introduction of the IL-15RF enabled expansion of iNK cells without addition of soluble cytokine and greatly improved longevity and functional persistence of iNK cells both in vitro and in animal models. Moreover, iNK cells modified with IL-15RF showed enhanced functional maturation, including upregulated expression of effector molecules such as granzyme B. iNK cells with both CD19-CAR and IL-15RF resulted in enhanced CAR functionality in vitro, and mouse models for B cell malignancy demonstrated that treatment with iNK cells engineered with CD19-CAR and IL-15RF were curative against B cell lymphoma (p<0.002), when compared with iNK cells alone or iNK cells modified with CD19-CAR alone.
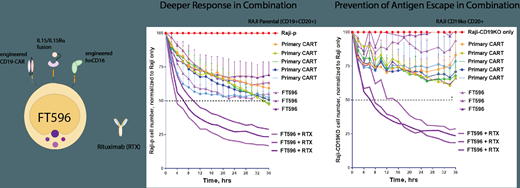
In combination with hnCD16, co-expression of CD19-CAR and IL15-RF culminates in iNK cells capable of dual-specificity through combinatorial use with monoclonal antibodies to tackle antigen escape. In long term killing assays, FT596 alone demonstrated equivalent levels of CD19 targeted anti-tumor activity as primary CD19-targeted CAR (CAR19) T cells when tested against CD19+ CD20+ B lymphoblast target cells and demonstrated enhanced levels of activity when used in combination with anti-CD20 (rituximab). When targeting CD19- CD20+ B lymphoblast target cells and used in combination with rituximab, only FT596 was able to effectively eliminate the CD19 antigen escaped target cell (64% vs 30% clearance of tumor cells at 36H vs rituximab alone). In vivo FT596 showed equivalent levels of tumor cell clearance as primary CAR19 T cells against the CD19+ acute lymphoblastic leukemia cell line NALM6 and CD19+CD20+ Burkitts lymphoma cell line RAJI, and enhanced clearance of RAJI tumor cells in combination with rituximab (p=0.0002). Furthermore, utilizing an allogenic human CD34 engrafted NSG mouse model, FT596 demonstrated improved survival and safety over primary CAR19 T cells, either as a monotherapy or as a combination therapy with rituximab versus RAJI tumor cells.
Together, these studies demonstrate FT596 provides a multi-antigen targeting, potent and persistent engineered immune cell that is derived from a master iPSC line which utilizes the intrinsic versatility of NK cells to enable a highly effective combination therapy in a single, standardized, scalable, off-the-shelf platform and supports the rational for a first-of-kind Phase I Study as a monotherapy and in combination with CD20-targeted mAbs including rituximab in subjects with relapsed/refractory B-cell lymphoma and leukemia.
Goodridge:FATE THERAPEUTICS: Employment. Mahmood:Fate Therapeutics, Inc: Employment. Gaidarova:Fate Therapeutics, Inc: Employment. Bjordahl:Fate Therapeutics, Inc.: Employment. Cichocki:Fate Therapeutics, Inc: Research Funding. Chu:FATE THERAPEUTICS: Employment. Bonello:Fate Therapeutics, Inc.: Employment. Lee:Fate Therapeutics, Inc.: Employment. Groff:FATE THERAPEUTICS: Employment. Meza:FATE THERAPEUTICS: Employment. Malmberg:Vycellix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics, Inc.: Consultancy, Research Funding. Miller:Moderna: Membership on an entity's Board of Directors or advisory committees; Dr. Reddys Laboratory: Membership on an entity's Board of Directors or advisory committees; CytoSen: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics, Inc: Consultancy, Research Funding; OnKImmune: Membership on an entity's Board of Directors or advisory committees; GT BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kaufman:FATE Therapeutics: Consultancy, Research Funding. Valamehr:Fate Therapeutics, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal