Azacytidine (AZA), the mainstay of therapy in high risk Myelodysplastic syndromes (HR-MDS), affects CD4+ T-cell polarization and function, but the effect of these changes on tumor immunity is unclear. Signal transducer and activator of transcription (STAT) proteins are key regulators of differentiation and polarization of CD4+ T-cells in both health and cancer, but the STAT signaling architecture of CD4+ T-cell subsets in HR-MDS and its modulation by AZA are currently unknown.
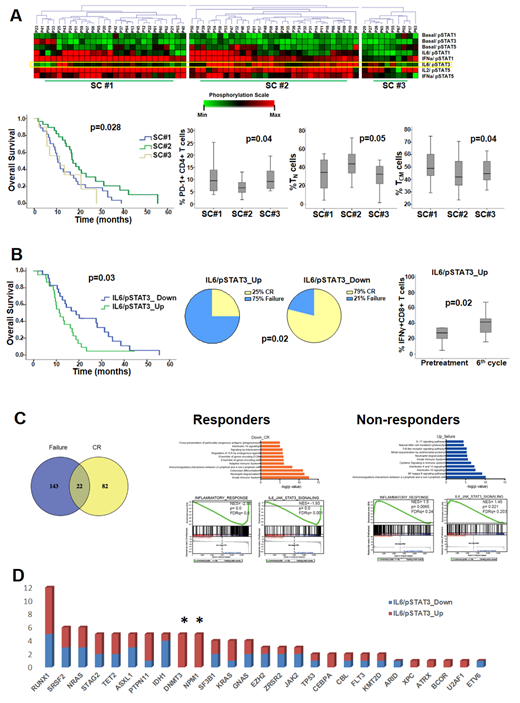
We applied single-cell phosphospecific flow cytometry in peripheral blood mononuclear cells from 67 HR-MDS patients at various time-points during AZA therapy. Unsupervised clustering of pretreatment STAT signaling profiles (SPs) of CD4+ T-cells revealed three signaling clusters (SCs), mainly differing in the potentiated responses of STAT3 to IL-6 stimulation (IL-6/STAT3 node). Compared to SC#1 and SC#3, patients in SC#2 displayed higher IL-6/STAT3 levels, higher levels of naïve (TN, p=0.05) and lower levels of PD1+ (p=0.04) and central memory ( TCM, p=0.04) CD4+ T-cells, and longer median overall survival (mOS, p=0.028, fig 1A). Moreover, comparisons of single signaling nodes revealed that the IL-6/STAT3 node correlated inversely with PD1+ (p=0.02) and IL-4+ (p=0.04) and positively with naïve CD4+ (p=0.04) and IFNγ+CD8+ T-cells (p=0.01). No other differences in clinicobiologic parameters, CD4+ and CD8+ T-cell subpopulations (FOXP3, IFNγ, IL-4, IL-17, Perforin and Helios) were noted among the 3 SCs and all other single nodes. To assess the effect of AZA on STAT signaling, we clustered the fold fold-change of pre- versus 6-month post-AZA SPs in CD4+ T-cells. Again the IL6/STAT3 node was the only differentiator among the clusters, and, by single node analysis, downregulation of IL6/STAT3 at 6th cycle (n=26) was associated with better response to AZA (p=0.02) and longer mOS (p=0.03), compared to upregulation of the same node (n=22); the latter also accompanied by an increase of IFNγ+CD8+ cells after AZA, (p=0.02, fig 1B). Further supporting a direct and beneficial modulation of the IL-6/STAT3 axis in CD4+ T-cells by AZA, the kinetics of IL-6/STAT3 during AZA therapy revealed a marked downregulation of the former node both at day15 (p=0.04) and cycle 6 after AZA (p=0.04) in responders (n=5), while no changes were observed in non-responders (n=7).
We further compared the transcriptional profiles of isolated bone marrow CD4+ T-cells between responders (n=4) and non-responders to AZA (n=4) by RNA-seq, both prior and after AZA. No significant differences in pretreatment gene expression were identified. By contrast, 105 genes were differentially expressed at cycle 6 compared to pretreatment in responders (FDR<0.2) and 145 genes in non-responders. Gene set enrichment (GSEA) revealed a significant downregulation of the IL-6/STAT3 pathway and the overall inflammatory response after AZA in responders, but a marked upregulation in non-responders, confirming the flow cytometry results (fig 1C).
To trace the molecular background associated with the differential regulation of the IL-6/STAT3 pathway we constructed mutational profiles by targeted DNA sequencing of 156 genes in blood mononuclear cells. No associations were found between pre or post-treatment IL-6/STAT3 node and mutational burden. By contrast, mutations in RNA splicing and STAG2 correlated with lower (p=0.02) and higher (p=0.017) pretreatment levels of IL6/STAT3, respectively. Notably, all 5 patients with NPM1/DNMT3A double mutation upregulated significantly IL6/STAT3 after AZA (p=0.03, fig 1D).
Collectively, our results reveal for the first time that downregulation of the IL-6/STAT3 signaling axis in CD4+ T cells may represent an immune-mediated mechanism of action of AZA. However, the antileukemic activity of IL-6/STAT3LowCD4+ T-cells appears to be independent from modulation of common metrics of tumor immunity, as, paradoxically, the detrimental IL-6/STAT3 upregulation was linked with an expansion of antitumor T cell subsets and decrease of the immunosuppressive ones. The IL-6/STAT3 axis is notoriously pro-tumorigenic and pharmacologic inhibition of various individual modules of this pathway in cancer is under development. Our findings may serve as a guidepost for the ongoing investigation of IL-6/STAT3 axis inhibition as a therapeutic strategy to overcome azacytidine resistance in HR-MDS.
Vassilakopoulos:Celgene / GenesisPharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; WinMedica: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pappa:Gilead: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene / GenesisPharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding. Papaemmanuil:Celgene: Research Funding. Kotsianidis:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal