Background
Interferons are recognized as active agents in the treatment of patients with high risk essential thrombocythemia (ET) or polycythemia vera (PV), both in the upfront setting as well as beyond. Several trials have shown high rates of hematologic and molecular responses with the use of interferons, however, data on direct comparison of interferon activity in patients with early disease in comparison to patients refractory or resistant to prior therapies, such as hydroxyurea (HU) are lacking. We conducted a controlled analysis of the activity of pegylated interferon alfa-2a (PEG) in two prospective parallel clinical trials conducted in these two unique patient populations.
Methods
The MPD-RC 111 (NCT01259817) was an international, multicenter, phase 2 open-label clinical trial that evaluated PEG therapy in patients with high risk PV and high-risk ET who were either refractory or intolerant (R/I) to HU by modified ELN criteria. The MPD-RC 112 trial (NCT01258856) enrolled patients with high risk ET/PV who were treatment-naïve (TN) (HU <3 months) and randomized them (1:1) to PEG or HU. All patients randomized to PEG were included in this analysis. Both protocols were conducted concurrently at the MPD-RC member institutions and utilized a similar primary endpoint of overall response rate (complete and partial response rates) by ELN Criteria at 12 months confirmed by the same blinded central review committee. Both studies utilized the same PEG starting dose of 45 mcg weekly and was titrated for response to a maximum of 180 mcg weekly. Secondary endpoints included safety information, impact on disease biomarkers, bone marrow (BM) response, and quality of life data.
Results
Patients
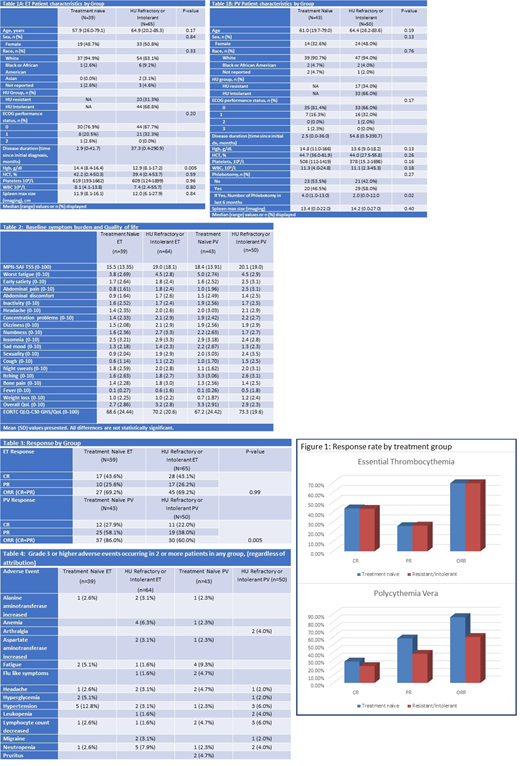
ET: 39 TN and 65 R/I ET patients were available for this analysis. Median disease duration was 2.9 months in TN and 37.3 months in R/I patients. Baseline characteristics and demographics were similar in the two cohorts except lower baseline hemoglobin level in RI patients. (Table1A)
PV: 43 TN and 50 R/I PV patients were included. Median disease duration was 2.5 months in TN and 54.8 months in R/I patients. Baseline characteristics only differed by lower frequency of phlebotomy rate in R/I patients. (Table1B) Baseline symptoms scores and quality of life were similar in TN and RI groups (Table 2)
Response
ET: CR/PR/ORR at 12 months were observed in 43.1%/26.2%/69.2% in R/I ET patients and in 43.6%/25.6%/69.2% in TN ET patients (p=0.99 for ORR). (Table 3, Figure 1)
PV: CR/PR/ORR at 12 months were observed in 22%/38%/60% in R/I PV patients, and in 27.9%/58.1%/86% in TN PV patients (p=0.005 for ORR). (Table 3, Figure 1)
Safety
PEG was equally well tolerated throughout both treatment groups with treatment discontinuation due to adverse events occurring in 14.6% in TN patients and 13.9% in R/I patients. The mean (SD) dose of PEG was 102.7 (52.3) mcg in R/I ET patients and 128.7mcg (46.4) in R/I PV patients. For TN patients, mean dose was 85.7mcg (59.7) in ET and 93.5 mcg (44.0) in PV. Adverse events were consistent with historic reports of PEG use and the distribution of events was similar in R/I and TN patients. (Table 4)
Conclusion
This intention to treat response analysis included TN and R/I ET and PV patients with balanced baseline characteristics who received prospective therapy with PEG. Patients with ET had a higher overall response rate at 12 months that was equivalent in patients who were treatment-naïve and in patients who were intolerant or refractory to HU. By contrast, patients with PV who were treatment-naïve had a higher ORR than patients those intolerant or refractory to HU. We conclude that treatment with PEG is an effective therapeutic option both treatment naïve PV and ET as well as those previously treated with HU, however PEG as a second line agent is especially effective in ET patients.
Yacoub:Hylapharm: Equity Ownership; Agios: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Ardelyx: Equity Ownership; Cara: Equity Ownership; Dynavax: Equity Ownership. Mascarenhas:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Roche: Consultancy, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merus: Research Funding. Mesa:AbbVie: Research Funding; Samus: Research Funding; Incyte: Research Funding; Sierra Onc: Consultancy; Genotech: Research Funding; Promedior: Research Funding; Novartis: Consultancy; Celgene: Research Funding; CTI Biopharma: Research Funding; La Jolla Pharma: Consultancy. Rampal:Agios, Apexx, Blueprint Medicines, Celgene, Constellation, and Jazz: Consultancy; Constellation, Incyte, and Stemline Therapeutics: Research Funding. Silver:PharmEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. McMullin:Daiko Sanyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Italopharma: Membership on an entity's Board of Directors or advisory committees. Ewing:Novartis: Honoraria, Other: Meeting attendance sponsorship ; Bristol Myers-Squibb: Other: Meeting attendance sponsorship . O'Connell:Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Shionogi: Membership on an entity's Board of Directors or advisory committees. Mead:Bristol Myers-Squibb: Consultancy; Novartis: Consultancy, Honoraria, Other: Travel/accommodation expenses, Research Funding, Speakers Bureau; CTI: Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Pfizer: Consultancy. De Stefano:Alexion: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Baer:Astellas: Research Funding; Al Therapeutics: Research Funding; Abbvie: Research Funding; Incyte: Research Funding; Forma: Research Funding; Kite: Research Funding; Takeda: Research Funding. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kremyanskaya:La Jolla: Consultancy; Incyte, Celgene, Constellation, Protagonist.: Research Funding. Hexner:novartis: Research Funding. Rambaldi:Amgen: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Italfarmaco: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau, travel support. Ritchie:Genentech: Other: Advisory board; Tolero: Other: Advisory board; agios: Other: Advisory board; Pfizer: Other: Advisory board, travel support; Celgene: Other: Advisory board; Jazz Pharmaceuticals: Research Funding; Celgene, Novartis: Other: travel support; AStella, Bristol-Myers Squibb, Novartis, NS Pharma, Pfizer: Research Funding; Ariad, Celgene, Incyte, Novartis: Speakers Bureau; Celgene, Incyte, Novartis, Pfizer: Consultancy. Kiladjian:Novartis: Honoraria, Research Funding; AOP Orphan: Honoraria, Research Funding; Celgene: Consultancy. Harrison:Promedior: Honoraria; Incyte: Speakers Bureau; Sierra Oncology: Honoraria; Celgene: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; CTI: Speakers Bureau; AOP: Honoraria; Shire: Speakers Bureau; Roche: Honoraria; Gilead: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Hoffman:Merus: Research Funding.
Pegylated Interferon Alfa-2a for in Patients with Polycythemia Vera or Essential Thrombocythemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal