Introduction: The progression of polycythemia vera (PV) to myelofibrosis (MF) is associated with significant morbidity and mortality. Interferon alfa (rIFNa), a disease-modifying agent, has potential to delay or prevent post-PV MF and improve overall survival but supporting data are required. We present results of the largest study demonstrating improved myelofibrosis-free and overall survival (MFS and OS) of rIFNa treated PV patients (pts) compared to other PV pts.
Objectives: To estimate the MFS and OS of treated PV pts and determine the relative risk for post-PV MF and mortality of those treated with rIFNa compared to those treated with other standard therapy.
Methods and Study Design: To ensure sufficient follow-up for analysis of long-term outcomes, this IRB approved study identified all adult pts treated at our center from 1974-2019 according to PVSG criteria (1974-2007), our published criteria (2008-2016) and WHO criteria (2016-2019) using a standardized query of electronic medical records. Demographic data, clinical characteristics, treatment history and outcomes were collected. The extended follow-up of this large PV cohort permitted us to evaluate the effectiveness of PV therapy using both intention-to-treat (ITT) and treatment duration (on-treatment) analyses. In the ITT analysis, pts were assigned to rIFNa or hydroxyurea (HU) arms according to the first cytoreductive treatment they received for at least one year or to phlebotomy only (PHL-O) if no cytoreductive treatment was given. On-treatment analysis was performed to account for cross-over and assess how duration of a given therapy influenced outcomes.
The onset of post-PV MF was defined by IWG-MRT criteria. MFS and OS were estimated using Kaplan-Meier methods and the log-rank test compared survival between treatment arms in ITT analysis. Multivariate analysis of post-PV MF risk and mortality was performed using a Cox proportional hazards model. The model accounts for age at diagnosis and is stratified by treatment arms (ITT) or by treatment as a time-dependent covariate (on-treatment).
Results: We identified 306 PV pts whose median age at diagnosis was 54 years (yr) (range 20-91) and of whom 151 (49%) were women. The median follow-up was 11 yr (range 1-45). The first line treatment was rIFNa in 75 (25%), HU in 134 (44%) or other cytoreductive regimens in 37 (12%). PHL-O was instituted in 60 pts (20%). Treatment cross-over occurred in 82 pts (27%), with the least from rIFNa arm (22%) (Table2). Treatment arms differed by age at diagnosis with a median of 50, 59 and 52 years for rIFNa, HU and PHL-O (p <0.01) (Table 3).
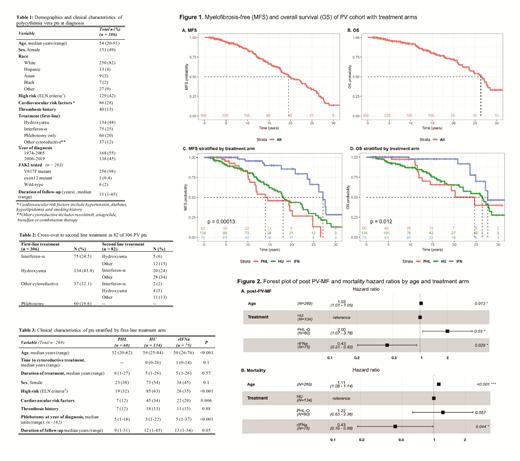
The median MFS and OS was 19.5 and 26.3 yr for the entire group; 27 and 28 yr for rIFNa arm; 18 and 26 yr for HU arm; and 14 and 25 yr for PHL-O (log-rank p<0.01 for MFS and p=0.01 for OS) (Figure 1). In multivariate analysis that included age, rIFNa arm had a lower risk of post-PV MF or death compared to HU arm (HR 0.43, p=0.03 and HR 0.44, p=0.04 respectively) and to PHL-O arm (HR 0.22, p<0.01 and HR 0.35, p=0.03 respectively) (Figure 2). The PHL-O arm had a higher risk of post-PV MF compared to HU as well. Older age at diagnosis was a risk factor for post-PV MF and death.
Accounting for cross-over, 138 pts received rIFNa at any time for a cumulative of 980 patient-years (median: 5.3, range 1-25 yr). On-treatment analysis associated rIFNa with an 8% and 7% relative risk reduction of post-PV MF and all-cause mortality respectively (age-adjusted HR of 0.92, p<0.01 and 0.93, p=0.01).
Discussion: This is the largest study with the longest follow-up of rIFNa treated PV pts and the first to demonstrate that rIFNa yields superior MFS and OS compared to HU or PHL-O. This study addresses the critical issue that randomized controlled trials to date failed to answer owing to limited follow-up duration and lack of surrogate endpoints for survival. Although the median age of the entire group is younger than the reported median age at PV diagnosis, multivariate analysis showed that both the survival benefit of rIFNa and the reduced risk of fibrosis are independent of age. This study supports early use of rIFNa for PV, especially in younger patients who should not be deprived of a disease-modifying therapy for being "low risk" by consensus criteria.
Conclusions: rIFNa yields improved MFS and OS of PV patients, independent of age, in this large study with extended follow up. Early use of rIFNa should be considered routinely in the management of PV.
Ritchie:agios: Other: Advisory board; Tolero: Other: Advisory board; Genentech: Other: Advisory board; Celgene, Incyte, Novartis, Pfizer: Consultancy; Ariad, Celgene, Incyte, Novartis: Speakers Bureau; Jazz Pharmaceuticals: Research Funding; Celgene, Novartis: Other: travel support; AStella, Bristol-Myers Squibb, Novartis, NS Pharma, Pfizer: Research Funding; Celgene: Other: Advisory board; Pfizer: Other: Advisory board, travel support. Silver:PharmEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal