BACKGROUND
Outcomes for patients (pts) with primary CNS diffuse large B cell lymphoma (PCNSL) have improved over recent years, largely through optimisation of first-line methotrexate (MTX)-containing protocols and dose-intensive chemotherapy consolidation. However, 30-50% of pts experience refractory or relapsed (r/r) disease, which confers very poor outcomes and short survival. By contrast to systemic DLCBL, there is no accepted standard approach for r/r PCNSL. Thiotepa is an alkylating agent highly efficient at crossing the BBB and widely incorporated within high-dose therapy and autologous stem cell transplantation (HDT-ASCT) protocols for PCNSL, but has not undergone dose-finding studies nor been incorporated within salvage regimens for PCNSL. The TIER study investigates the safety and efficacy of adding thiotepa, in a dose-escalation design, to the Rituximab, Ifosphamide and Etoposide (R-IE) salvage regimen1.
METHODS
TIER is an open label, phase I/II UK NCRI TAP study for pts with r/r PCNSL, previously treated with a high-dose MTX-based regimen. In phase I, the RP2D of thiotepa within the TIER combination was established using a 3+3 design, with dose escalations of 30, 40 and 50mg/m2 (dose level 2 (n=4), 3 (n=5) and 4 (n=27) respectively), given on day 5 of R-IE cycle1. The Phase II primary endpoint was overall response rate (ORR) after cycle 2 of TIER (C2) by centrally reviewed contrast-enhanced MRI2, on an intention-to-treat (ITT) basis. Further treatment and consolidation after the primary endpoint MRI was at the discretion of investigators. Key secondary end-points were 2-year PFS, EFS and OS.
RESULTS
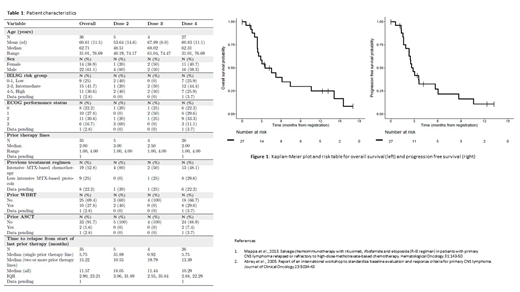
Thirty-six pts were recruited from Jun 2015-Apr 2019 at 13 centres (characteristics: Table 1). The median number of prior lines was 2 (range 1-4) with 44% of patients deemed refractory to their previous line of therapy. During phase I (n=10) no dose limiting toxicities (DLTs) were observed up to and including 50mg/m2 thiotepa, constituting the RP2D (n=27). 56 cycles of TIER were administered across both phases; 5 pts had >1 dose reductions. Median time between C1D1 and C2D1 was 24.7 days (range 22-38). Relative mean dose intensity of thiotepa, ifosphamide and etoposide was 71.8 (SD 44.9), 76.4 (SD 41.6), and 76.8 (SD 41.8) respectively. Further therapy after 2 cycles of TIER was delivered to 41.7% of pts (16.7% ASCT, 22.2% TIE, and 2.8% WBRT).
The most common grade 3 / 4 adverse events were thrombocytopenia and neutropenia (47.2 and 55.6% occurring in 15 and 18 pts respectively). 17 serious adverse events (SAEs) were reported in 12 pts, of which 4 were haematological. Of these, 13 were considered related to TIER. Commonly occurring non-haematological SAEs were respiratory tract infections (23.5%) and seizures (11.8%).
At data lock, 10/27 pts had responded to treatment as assessed by local investigators (ORR 37%, CR rate 15%). 13 pts were non-evaluable (7 progressed prior to C2 scan, 2 withdrew consent, and 4 awaiting scans) and classed as non-responders. Following data lock, responses were reported for 3 further pts (2 by local investigators and 1 by central review), resulting in a provisional ORR of 13/27 (48%). For the ITT population, median OS time was 3.52 months (95% CI 2.50, 14.17), and median PFS 2.86 months (95%CI 2.34, 6.05) (Figure 1). 6 pts underwent ASCT consolidation after TIER, of whom 2 have relapsed.
CONCLUSIONS
This is an early analysis of data from the phase I/II TIER study for a heavily pre-treated group of r/r PCNSL; the first such dose-escalation study for this patient group. Phase I determined a RP2D of 50mg/m2 of thiotepa incorporated in the R-IE regimen. There were no DLTs and toxicities, consistent with an intensive ifosphamide-based regimen. ORR was 10 pts (37%) with 3 further responses reported after data lock. If confirmed by central review, the primary endpoint of activity (ORR 40%) will have been reached. However, notwithstanding the response endpoint, most pts experienced very poor survival outcomes. These data describe the feasibility of TIER as a salvage regimen for r/r PCNSL but question the utility of this approach as a standard option, given the short PFS and OS times, except for the minority of pts who received ASCT. Biological and imaging sub studies are underway to identify predictors of outcome. In the modern era of effective first line immunochemotherapy approaches, pts with r/r PCNSL remain a major area of unmet need for whom novel experimental approaches are urgently required.
Fox:Gilead: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Sunesis: Consultancy; Takeda Pharmaceuticals: Consultancy; Atara Biotherapeutics: Consultancy; Adienne: Other: Travel Support. Martinez-Calle:ABBVIE: Other: Travel support. Collins:Gilead: Consultancy, Honoraria. Ferreri:Novartis: Consultancy; Celgene: Consultancy, Research Funding; Roche: Research Funding; Kite: Consultancy. Davies:BioInvent: Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Karyopharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Bayer: Research Funding; ADCT Therapeutics: Honoraria, Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; MorphoSys AG: Honoraria, Membership on an entity's Board of Directors or advisory committees. Johnson:Roche: Consultancy, Honoraria, Speakers Bureau; Takeda: Other: Travel, accomodations, expenses. Cwynarski:Adienne: Consultancy; Takeda: Consultancy, Other: conference and travel support , Speakers Bureau; Roche,: Consultancy, Other: conference and travel support, Speakers Bureau; Autolus: Consultancy; KITE: Consultancy; Gilead: Consultancy, Other: conference and travel support, Speakers Bureau; Celgene: Consultancy; Atara: Consultancy; Janssen: Other: conference and travel support, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal