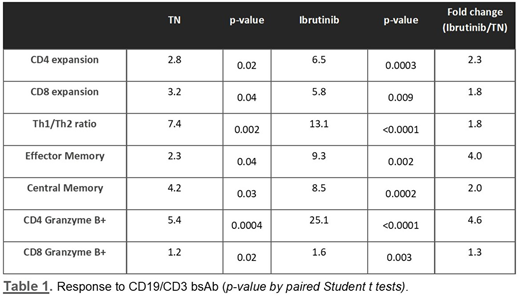
Targeting B-cell receptor signaling with ibrutinib, the first-in-class irreversible inhibitor of Bruton tyrosine kinase, has become a highly successful treatment modality for Chronic Lymphocytic Leukemia (CLL) patients. However, there remains a need for adjunct treatments to deepen responses and prevent or treat resistant disease. Ibrutinib also inhibits inducible T-cell kinase (ITK) which is hypothesized to improve antitumor T-cell immunity. We developed a CD19/CD3 bispecific antibody (bsAb) in a 100 kDa single chain-Fv-Fc format (19/3-scFv-Fc). We previously reported increased T-cell activation and cytotoxic activity by the bsAb in blood samples from ibrutinib-treated patients compared to treatment-naïve patients. To better understand how ibrutinib affects T-cell function and increases cytotoxic activity we assessed T-cell responses to the bsAb in samples from ibrutinib-treated patients (12 ± 2 months on ibrutinib) and treatment-naïve (TN) patients. We cultured peripheral blood mononuclear cells (PBMCs) from both patient groups with bsAb and measured CLL cell death, T-cell expansion, activation, and differentiation by flow cytometry. After 5 days of exposure, the CD19/CD3 bsAb induced superior killing of ibrutinib-treated CLL cells, with a median specific-killing of 97% for ibrutinib-treated compared to 77 % for treatment-naïve patient samples (n = 15; p= 0.008). In vitro treatment with CD19/CD3 bsAb induced ≥ 5-fold expansion of autologous CD8 and CD4 T cells and an increase of CD45RO+CCR7- effector memory and CD45RO+CCR7+ central memory CD4 T-cells in response to bsAb (Table 1). We also observed a shift towards Th1 (CCR6-CXCR3+) polarization, and higher T-cell granzyme B expression in response to CD19/CD3 bsAb. While T cells from both patient groups responded to the bsAb, all these effects were more pronounced in ibrutinib-treated samples compared to treatment-naïve samples (Table 1). There was a 13-fold increase of the Th1/Th2 ratio in PBMCs from ibrutinib-treated patients compared to a 7-fold increase in the treatment-naïve group. Moreover, the bsAb induced a significant increase of activation markers HLADR (p=0.0013; p=0.0006) and CD27 (p< 0.001; p=0.0114) on CD4 and CD8 T cells in the ibrutinib-treated patient samples, while there was no significant change in T-cell activation in the treatment-naïve group. Granzyme B expression in CD8 T cells was high even in untreated samples and its upregulation by the bsAb was comparable in both patient groups. However, we observed a 25-fold increase in granzyme B positive CD4 T cells in ibrutinib-treated samples, 5-fold higher than in the treatment-naïve samples.
In summary, the enhanced cytotoxic activity of T cells in response to the bsAb in samples from ibrutinib-treated patients correlated with increased memory cell differentiation, higher T-cell activation, and a strong shift towards a Th1 phenotype. As ibrutinib enhances anti-CLL activity of autologous T cells, bsAb immunotherapy may work well with concurrent ibrutinib treatment. These data support the investigation of bsAbs in combination with ibrutinib for immunotherapy of CLL. The mechanisms underlying the enhanced T-cell responses remain to be fully elucidated. In particular, it will be important to define whether inhibition of ITK by ibrutinib plays a role in enhancing T-cell dependent cytotoxicity.
Supported by the intramural program of the NHLBI/NIH.
Rader:NIH: Patents & Royalties: ROR1 mAb 2A2. Wiestner:Acerta: Research Funding; Merck: Research Funding; Nurix: Research Funding; Pharmayclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal