T-cell large granular lymphocytic leukemia (T-LGLL) is an incurable, and likely under-diagnosed leukemia characterized by abnormal clonal proliferation of CD8+ memory T-cells. The clonal outgrowth of T-LGLL cells can lead to the development of profound neutropenia and anemia which results in frequent infections, transfusion dependence, and impairment in quality of life and lifespan. There have been few prospective clinical trials in this disease, and no drugs have been FDA approved for its treatment. The primary driver of leukemogenesis in T-LGLL is known to be interleukin-15 (IL-15), a gamma-chain cytokine that induces proliferation of T-LGLL cells. BNZ-1 is a novel pegylated peptide antagonist that inhibits IL-15 by binding to the common γ-chain receptor for cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. Studies utilizing BNZ-1 in vitro on T-LGLL cell lines, and ex vivo on clinical patient samples demonstrated significant inhibition of downstream signaling and increased LGLL cell apoptosis (Wang et al., Leukemia 2018). Given these results, we conducted a phase I/II dose escalation study to evaluate the safety, maximum tolerated dose (MTD), and preliminary efficacy of BNZ-1 in T-LGLL (NCT03239393).
Patients with T-LGLL were eligible if they had one or more of the following: absolute neutrophil count (ANC) <500 cells/m3, neutropenia with recurrent infections, or symptomatic or transfusion-dependent anemia. Diagnosis of T-LGLL required: >400/mm3 CD3+CD57+ cells or >650 mm3 CD8+ cells, with a clonal T-cell receptor rearrangement. No prior therapy within 30 days or 5 half-lives was permitted. MTD was evaluated using a standard 3+3 design; with a dose escalation strategy using four doses of BNZ-1: 0.5 mg/kg, 1 mg/kg, 2 mg/kg, and 4 mg/kg. BNZ-1 was administered by infusion on Days 1, 8, 15, and 22 of a 4-week cycle. Patients then had the option to enter the 3-month extension period, at the same weekly dose. Efficacy was determined utilizing criteria from the ECOG5998 study in T-LGLL. CR was defined as complete normalization of blood counts. Partial response (PR) in neutropenic patients was determined by 4 weeks or greater response with ANC >500 cells/mm3 if >/=50% improvement from baseline. For transfusion-dependent anemia patients, a >/=50% decrease in monthly transfusions for at least 2 months was required for a PR. For patients with symptomatic anemia, improvement in hemoglobin >/=1 g/dL with improvement in symptoms constituted a PR. Patients with a response were permitted to remain on a long-term extension (LTE).
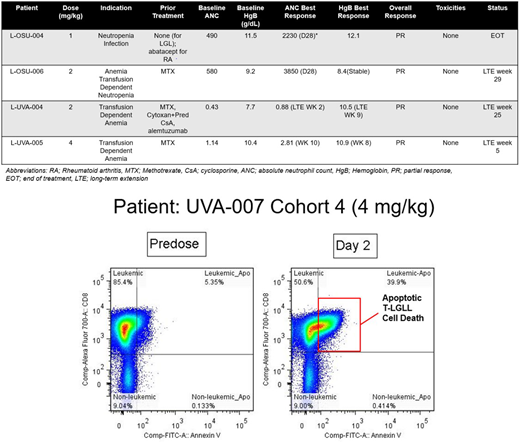
Eighteen patients, at 3 US centers were enrolled on study including: 3 patients at 0.5 mg/kg, 4 at 1 mg/kg, 5 at 2 mg/kg, and 6 at 4 mg/kg. 10 patients were enrolled for neutropenia, 4 for transfusion dependent anemia, 2 for symptomatic anemia, and 2 with anemia and neutropenia. 15 patients (83%) completed all 16 weeks of treatment, 2 patients declined to enter the extension phase, and one patient on the 2 mg/kg dosage was taken off study at 4 weeks due to neutropenia <100 thought secondary to T-LGLL. One patient developed grade 2 hyperbilirubinemia, which was thought possibly due to study drug though was grade 1 at baseline.The MTD was not reached. Four patients attained a PR: 3 patients with transfusion-dependent anemia became transfusion independent, while one patient with neutropenia had significant resolution of her neutropenia (Table). These three patients remain on the LTE, though one patient is under observation. Correlative studies demonstrated apoptosis of T-LGLL cells on flow cytometry utilizing CD3 T-cell gating within 24 hours of the first dosage of BNZ-1 (a representative example is shown in the Figure), confirming in patients that inhibition of IL-15 induces apoptosis of T-LGLL cells.
In this Phase I/II clinical trial, IL-15 blockade utilizing BNZ-1 demonstrated increased apoptosis in patients with T-LGLL, with early evidence of clinical response, particularly amongst patients with transfusion-dependent anemia. Remarkably, these patients remained transfusion-independent while on BNZ-1. The MTD was not reached in this cohort of patients, and there were minimal AEs associated with BNZ-1. Further analysis of responding patients is underway to determine the most effective approach utilizing BNZ-1 in this rare disease.
Brammer:Celgene: Research Funding; Seatlle Genetics: Honoraria, Speakers Bureau. Sokol:EUSA: Consultancy. Tagaya:Bioniz: Membership on an entity's Board of Directors or advisory committees; Bioniz: Research Funding. Rogers:AbbVie: Research Funding; Acerta Pharma: Consultancy; Genentech: Research Funding; Janssen: Research Funding. Waldmann:Bioniz: Membership on an entity's Board of Directors or advisory committees. Azimi:Bioniz: Employment. Frohna:Bioniz: Employment. Ratnayake:Bioniz: Employment. Loughran:Bioniz: Membership on an entity's Board of Directors or advisory committees; Keystone Nano: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal