Background:FLT3-internal tandem duplication (FLT3-ITD) mutation is a poor prognostic factor for acute myeloid leukemia (AML). Several second-generation FLT3-targeted tyrosine kinase inhibitors with high selectivity and potency have been developed to date. Recently, gilteritinib was approved for the FLT3 mutation-positive relapsed or refractory AML patients. However, acquired mutations at the F691 residue in FLT3 kinase domain were identified in the patients who had disease progression after the treatment with gilteritinib, as with quizartinib treatment that caused resistant mutations at F691 and D835 residues. Therefore, it is very important to clarify the potencies of each FLT3 inhibitors against acquired FLT3 mutations for clinically selecting an appropriate FLT3 inhibitor according to the mutation type. In this study, we explored the resistant mutations against FLT3 inhibitors by random mutagenesis analysis and evaluated the cross-reactivity of FLT3 inhibitors against each resistant mutation.
Methods: For random mutagenesis assay, 32D cells were infected with retroviruses encoding randomly mutagenized human FLT3-ITD. FLT3-ITD dependent 32D cells were established without an addition of IL-3, and then treated with FLT3 inhibitors, gilteritinib, FF-10101 and quizartinib, at concentrations of GI95 and 3 x GI95. Two weeks after treatment, full length FLT3-ITD sequences of viable clones were analyzed. The identified mutated FLT3-ITDs were introduced into 32D cells to confirm the resistance to FLT3 inhibitors. For cell growth assay, 32D transfectants were incubated with 5 FLT3 inhibitors (gilteritinib, FF-10101, quizartinib, crenolanib and midostaurin) for 3 days followed by determination of cell viability.
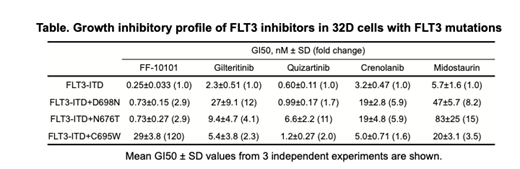
Results: We identified the gilteritinib-resistant mutation (FLT3-ITD+D698N), quizartinib-resitant mutation (FLT3-ITD+N676T), and FF-10101-resistant mutation (FLT3-ITD+C695W) (Table). Inhibitory activity of gilteritinib against FLT3-ITD+D698N-expressing 32D cells (GI50, 27 nM) was decreased by 12-fold as compared with that against original FLT3-ITD-expressing 32D cells (GI50, 2.3nM). FF-10101 (GI50, 0.73 nM), quizartinib (GI50, 0.99 nM) and crenolanib (GI50, 19 nM) retained potency against FLT3-ITD+D698N, while midostaurin (GI50, 47 nM) did not have a potency. In FLT3-ITD+N676T-expressing 32D cells, inhibitory activities of quizartinib and midostaurin were decreased by 11 and 15-fold (GI50, 6.6 nM and 83 nM, respectively), while FF-10101 (GI50, 0.73 nM), gilteritinib (GI50, 6.6 nM) and crenolanib (GI50, 19 nM) retained potency. In FF-10101-resistant mutation (FLT3-ITD+C695W)-expressing 32D cells, the other inhibitors retained growth inhibitory activity.
Conclusions: Resistant mutations to gilteritinib and quizartinib were newly identified in FLT3 kinase domain by random mutagenesis analysis. FF-10101 retained potent inhibitory activities against FLT3-ITD+N676T conferring resistance to quizartinib and midostaurin, and FLT3-ITD+D698N resistant to gilteritinib and midostaurin, although FF-10101 was vulnerable to FLT3-ITD+C695W substituted for C695 residue which forms covalent bond with FF-10101. These results indicated that FF-10101 was a promising agent for the treatment of patients with AML with FLT3 inhibitor-resistant mutations newly identified in this study.
Ishikawa:Bristol-Myers Squibb: Honoraria; Abbvie GK.: Honoraria; Celgene Co., Ltd.: Honoraria; Kyowa Hakko Kirin Co., Ltd.: Honoraria. Saito:FUJIFILM Corporation: Employment. Morimoto:FUJIFILM Corporation: Employment. Murao:FUJIFILM Corporation: Employment. Terada:FUJIFILM Corporation: Employment. Yamaura:FUJIFILM Corporation: Employment. Hagiwara:FUJIFILM Coporation: Employment. Kiyoi:Daiichi Sankyo Co., Ltd: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; FUJIFILM Corporation: Research Funding; Pfizer Japan Inc.: Honoraria; Bristol-Myers Squibb: Research Funding; Perseus Proteomics Inc.: Research Funding; Otsuka Pharmaceutical Co.,Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Astellas Pharma Inc.: Honoraria, Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal