Background: Overexpression of anti-apoptotic proteins such as Bcl-2, Bcl-xL and Mcl1 is commonly detected in acute myeloid leukemia (AML) and is correlated with poor patient prognosis. Fortunately, in most AML samples, the expression of pro-apoptotic proteins, including Bax, Bak and Bim, is also increased. This is likely due to a feedback mechanism, which results in AML cells that are particularly vulnerable to treatment with targeted inhibition of anti-apoptotic proteins. However, challenges arise when the important pro-survival proteins named above are either poorly targeted by specific inhibitors (such as BH3-mimetics), as in the case of Mcl1, or when the use of these inhibitors results in severe thrombocytopenia, as in the case of Bcl-xL. Therefore, the development of novel medications to target such molecules with high specificity and low toxicity is of crucial importance.
GEX1A, a splicing modulator, induces a large shift in the pattern of exon skipping and intron retention events by inhibiting the SF3B1-PHF5A complex of the U2 snRNP. While GEX1A has shown pre-clinical efficacy in some carcinoma cell lines containing various spiceosomal mutations, the in vitro and in vivo effects of GEX1A in leukemic cells which lack spiceosomal mutations are largely unknown.
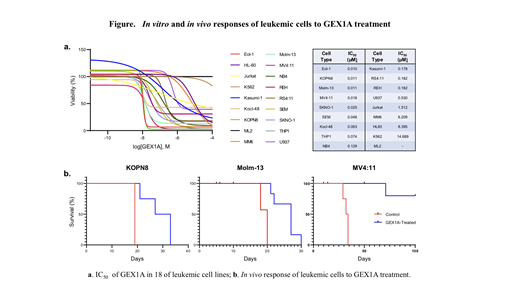
Experimental procedures: The IC50 of 18 established AML cell lines was determined by treating the cells with GEX1A in vitro using a series of 10-fold dilutions; cell viability was then examined using quantitative colorimetric assays. The in vivo anti-leukemic efficacy of GEX1A was evaluated using xenograft models generated in NSG mice injected with the GEX1A-sensitive human AML cell lines KOPN8, Molm-13, and MV4:11. The molecular mechanism by which GEX1A kills AML cells was then studied.
Results: Based on their IC50, 18 established leukemic cell lines can be divided into high (LD50 < 100 nM), moderate (LD50 = 100-500 nM), and low sensitivity (LD50 > 500 nM) groups. We next determined the tolerable dose of GEX1A for in vivo mouse treatment. Intraperitoneal injection of GEX1A significantly improved the survival of leukemic mice compared to the vehicle control group (see Figure). Mechanistically, we found that GEX1A kills leukemic cells by inducing the production of pro-apoptotic Mcl1-S protein at the expense of the pro-survival Mcl1-L protein due to the alternative splicing of the Mcl1 gene. In addition, we found that high levels of Bcl-xL protein predict resistance of leukemic cells to GEX1A treatment. Furthermore, synergistic activity was observed in 2/6 cell lines treated with GEX1A and ABT-263, an inhibitor of Bcl-2/Bcl-xL/Bcl-w, and 6/6 cell lines treated with GEX1A and A-1155463, a highly potent Bcl-xL inhibitor.
Conclusion: GEX1A is a novel splicing modulator that shows potent anti-leukemic activity. GEX1A kills leukemic cells by inducing a splicing isoform switch of the Mcl1 gene to produce a pro-apoptotic Mcl1-S protein at the expense of the pro-survival Mcl1-L protein in such cells. GEX1A and a Bcl-xL-specific inhibitor were also combined to show synergistic effects on leukemic cells. Our results indicate that GEX1A may be an effective treatment for leukemic patients when combined with a specific Bcl-xL inhibitor. We are actively evaluating our combination treatment using primary patient samples in in vivo xenograft models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal