
BACKGROUND: Allogenic hematopoietic stem cell transplant (SCT) is a potentially curative option for patients with acute myeloid leukemia (AML). However, patients who are older, are ineligible for intensive chemotherapy due to significant comorbidities, or have biologically aggressive and refractory disease are not often considered as viable SCT candidates. Venetoclax, a highly selective, potent BCL-2 inhibitor that induces apoptosis in AML cells, has resulted in high rates of durable remission when combined with hypomethylating agents (HMAs) or low dose cytarabine (LDAC), in older patients traditionally considered high risk and ineligible for standard induction chemotherapy. Here, patients who received SCT after venetoclax-based treatments were analyzed to determine the impact of SCT on patient outcomes.
METHODS: This study includes patients from the global, open-label phase 1b (NCT02203773) and phase 1/2 (NCT02287233) clinical trials studying the safety and efficacy of venetoclax in combination with the HMAs decitabine or azacitidine, and LDAC, respectively. Patients had newly diagnosed AML and were ineligible for intensive chemotherapy due to comorbidities or age. Patients in the venetoclax plus HMA trial (n=212) were treated with venetoclax (400, 800, or 1200 mg) coadministered with either 20 mg/m2 of intravenous decitabine on days 1-5 or 75 mg/m2 of intravenous or subcutaneous azacitidine on days 1-7 within each 28-day cycle. Patients in the venetoclax plus LDAC trial (n=92) were treated with venetoclax (600 or 800 mg), and with LDAC (20 mg/m2 daily) subcutaneously administered on days 1-10 of each cycle. Patients proceeding to SCT were evaluated for efficacy endpoints including best response (complete remission [CR] or CR with partial hematologic recovery [CRh]), time to best response, time from last dose of venetoclax until SCT, and 12-month post-SCT survival.
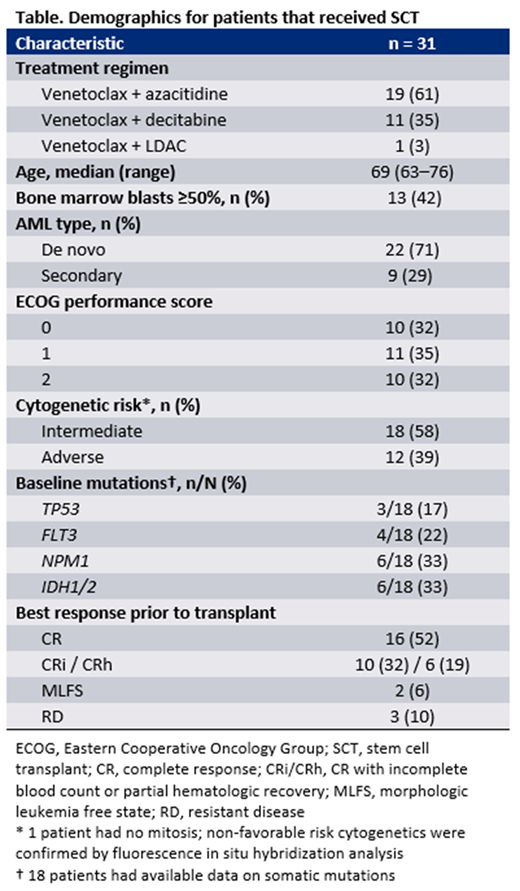
RESULTS: Of 304 patients treated with venetoclax-based therapy, 31 (10%) proceeded to have allogenic SCT, all of whom were treated in the US. Key demographics for those who received SCT are shown in the Table. The median age was 69 (range: 63-76), 29% had secondary AML, and 39% had adverse cytogenetic risk. The median time on study drug was 3.7 months (range: 0.9-20), and the median time from last dose of venetoclax to SCT was 1.2 months (range: 0.4-10). Twenty-two (71%) patients achieved CR or CRh, with a median time to best response of 2.3 months (range: 0.9-7.1). The median survival time for those that achieved CR (n=16) and CRh (n=6) was 28 months (range: 5.6-54) and 32 months (range: 14-41), respectively. Of 22 patients that achieved CR or CRh, 13 (59%) remained in remission for at least 12 months posttransplant. Across all patients, 55% (17/31) remained in remission for at least one year after SCT, with 12 such patients having remained in remission for ≥2 years. Overall, 68% (21/31) of patients remained alive at 12 months posttransplant.
CONCLUSIONS: Venetoclax in combination with either HMAs or LDAC has led to high rates of early, deep and durable responses in untreated AML patients ineligible for standard induction chemotherapy. In this combined study of over 300 such patients, 10% went on to receive stem cell transplant, a majority of whom remained alive and in remission for at least 12 months thereafter. Nearly 40% of SCT patients had durable remissions (≥2 years), suggesting that venetoclax-based regimens prior to SCT, even in patients deemed unfit for standard induction chemotherapy, may provide a path to curative therapy.
Pratz:Astellas: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium/Takeda: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding. DiNardo:abbvie: Consultancy, Honoraria; daiichi sankyo: Honoraria; celgene: Consultancy, Honoraria; agios: Consultancy, Honoraria; jazz: Honoraria; medimmune: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; syros: Honoraria. Arellano:Gilead: Consultancy. Letai:Zeno Pharmaceuticals, Vivid Bioscience, Flash Therapeutics, Dialectic Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Cofounder or Advisory Board member; AbbVie, AstraZeneca, Novartis: Consultancy, Research Funding. Thirman:Merck: Research Funding; Roche/Genentech: Consultancy; Gilead: Research Funding; TG Therapeutics: Research Funding; Up to Date: Honoraria; AbbVie: Consultancy, Research Funding; Astra Zeneca: Consultancy; Pharmacyclics: Research Funding; Celgene: Consultancy; Janssen: Consultancy. Roboz:Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees. Becker:The France Foundation: Honoraria; AbbVie, Amgen, Bristol-Myers Squibb, Glycomimetics, Invivoscribe, JW Pharmaceuticals, Novartis, Trovagene: Research Funding; Accordant Health Services/Caremark: Consultancy. Hong:Roche: Equity Ownership; Genentech Inc.: Employment, Equity Ownership. Jiang:AbbVie, Inc.: Employment, Other: stock or options. Hayslip:AbbVie, Inc.: Employment, Other: stock or options. Potluri:AbbVie, Inc.: Employment, Other: Stock/stock options. Pollyea:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Diachii Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal