Background
Although much efforts have been made to precisely define fitness of AML patients, in patients who are not candidate to chemotherapy, there is no prognostic model and the respective weight of AML biology and patient fitness are not well established. Here we test AML-CM score (Sorror, JAMA 2018), that is validated in fit population, in a set of old AML patients who received HMAs.
Methods
We retrospectively collected data of consecutive patients who received HMAs in our institution from 1st Jan 2008 with an age > 65 years at AML diagnosis. AML-CM score was applied to all the patients. Patients were divided in 4 groups (score 1-4: group 1, score 5-6: group 2; score 7-9: group 3, score > 9: group 4) and in 2 macro-groups (score 1-6: group A and score > 6 group B) for the analyses. Descriptive data are presented as median with interquartile ranges (IQR). Adverse events are graded according to CTCAE v4.03. Survival analysis was conducted with Kaplan-Meyer and are presented as 95% confidence intervals (C.I.) and differences in overall survival (OS) were tested with 2-side log rank test. Fisher exact test and Person's chi squared test were used whenever appropriate.
Results
At data cut-off, 1st Jan 2019, 60 consecutive patients received decitabine or azacytidine as 1st line therapy for AML. Median age of the population was 75.94 years (IQR 72.53-80.38). Most of the patients (37/62, 59.7%) had de novo AML, 19/62 (30.6%) had AML secondary to previous myeloid disorders and 6/62 (9.7%) had AML secondary to chemotherapy or radiotherapy. Most of the patients were smokers (19/33, 57.57%, 29 no data), and few were usual drinkers (4/16, 25.00%, 46 no data). In our set, out of 62 patients, 2 patients (3.2%) had inv(3), 1 (1.6%) a translocation involving 11q23, 1 (1.6%) del(5q), 4 (6.4%) mon(7) or del (7q), 1 (1.6%) del(17p), 15 (24.2%) complex karyotype, 27 (43.5%) normal karyotype, 4 (6.5%) other alterations and 5 were not evaluable; 3/17 (17.65%, 45 no data) harbored IDH2 mutation, 1/16 (6.25%) IDH2 mutation, 2/33 FLT3 mutation (6.06%, 29 no data), 1/24 (4.17%, 38 no data), 2/15 (13.33%, 47 no data) TP53 mutation. According to ELN 2017, 3/62 patients (4.83%) had low risk, 34/62 (54.84%) intermediate risk and 23/62 (37.10%) high risk AML.
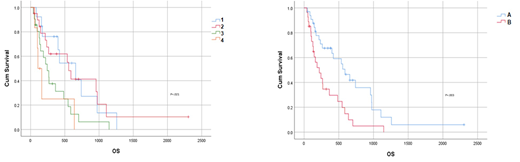
According to AML-CM score, 13/62 patients (20.97%) were in group A, 20/62 (32.36%) in group B, 21/62 (33.87%) in group C, 6/62 (9.68%) in group D, 2/62 (3.23%) were not allocated for incomplete AML-CM score. There was no difference in term of age, ELN risk, secondary AML prevalence, HMA administered, or response to HMA according to ELN criteria between group 1, 2, 3, 4 or between macro-group A and B. Cardiovascular comorbidity, diabetes mellitus, obesity, previous tumor, hypoalbuminemia, elevated LDH were prevalent in higher risk AML-CM groups (3-4) and in macro-group B. Median OS was 658 days (95% C.I. 316-1000) in group 1, 556 days (95% C.I. 463-649 in group 2, 243 days (95% C.I. 153-353) in group 3, 107 days (95% C.I. 47-167) in group 4 (p=.021, figure 1A). Furthermore, we observed a median OS of 589 days (95% C.I. 328-850) in macro-group A and 219 days (95% C.I. 96-342) in macro-group B (p=.003, figure 1B). Reduced survival was correlated with a non-statistical trend toward augmented incidence of infections and adverse events in higher risk AML-CM groups (3-4).
Conclusions
AML-CM is a useful indicator of prognosis in old patients that receive HMAs. Prognosis in our set is influenced by comorbidity (measured with AML-CM, a quantitative score) more than by disease biology. We identified a group of patients (macro-group A) that has median OS after HMAs outlying OS reported in literature. This brilliant result can be due to lower comorbidity. AML-CM could help in defining candidate patients for therapy intensification and care utilization or for team comorbidity management.
GM and RDN equally contributed
Martinelli:Roche: Consultancy; Novartis: Consultancy; ARIAD: Consultancy; BMS: Consultancy; Pfizer: Consultancy. Baccarani:Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Takeda: Consultancy. Papayannidis:Pfizer: Honoraria; Teva: Honoraria; Shire: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Incyte: Honoraria. Cavo:janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; novartis: Honoraria; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal