Background: For patients (pts) with AML undergoing induction chemotherapy (IC), incomplete platelet (plt) recovery can lead to an increased risk of bleeding, prolonged dependence on plt transfusions, plt alloimmunization, as well as delay in post-remission therapy. Eltrombopag is a thrombopoietin receptor agonist that stimulates megakaryopoiesis and has anti-leukemic effects. Herein, we present the final results of a phase 2 trial evaluating the efficacy of eltrombopag in accelerating plt count recovery in older AML pts receiving IC (NCT02071901).
Methods: All newly diagnosed FLT3-negative AML pts > 60 years (yrs) with ECOG scores of 0-2, no active second malignancy, and no evidence of marrow fibrosis at the time of AML diagnosis were included. Acute promyelocytic (AML-M3), megakaryocytic (AML-M7), and AML arising from myeloproliferative neoplasms were excluded. Pts with any arterial or venous thrombotic event (excluding line thrombosis) within the past 12 months were excluded. IC consisted of an anthracycline (daunorubicin at 45 mg/m2 x 3 days) and infusional cytarabine (100 mg/m2 x 7 days). Eltrombopag was administered starting day 15 (range, day 15-18) of IC in pts who had marrow blasts <5% on day 14 (range, day 14-17). The primary endpoint was the proportion of pts achieving plt count > 50000/µL by day 24 of IC. In a historical cohort of 220 older AML pts (excluding APL and AML-M7) treated with IC between 2004 and 2011 at Cleveland Clinic, 60% achieved a plt count > 50,000/µL by day 24. Eltrombopag was administered at a dose of 200 mg with a one-time maximal dose escalation to 300 mg daily if plts remained < 50,000/µL after 2 weeks on treatment. Eltrombopag was discontinued once plts exceeded 100,000/µL. Informed consent was obtained prior to start of IC, and eligibility was determined on day 14.
Results: Between 9-2014 and 9-2018, 93 pts were consented, of whom 31 met the eligibility criteria. Sixty seven percent of pts were ineligible as they had >5% blasts on day 14 marrow and the remaining met one or more of the following exclusion criteria: acute clinical worsening; use of supraphysiologic dose of steroids (n=1); marrow fibrosis (n=1); active second malignancy (n=1); elevated transaminases (n=5), and elevated serum creatinine (n=1). Median age was 67 years (range, 60-78), 38% were female, and 81% had a good ECOG status (ECOG 0 or 1). Most pts (84%) had de novo AML, 16% had secondary AML. By CALGB/Alliance criteria, 82% had intermediate cytogenetics, 9% had adverse karyotype and in 9% cytogenetic analysis was unsuccessful.
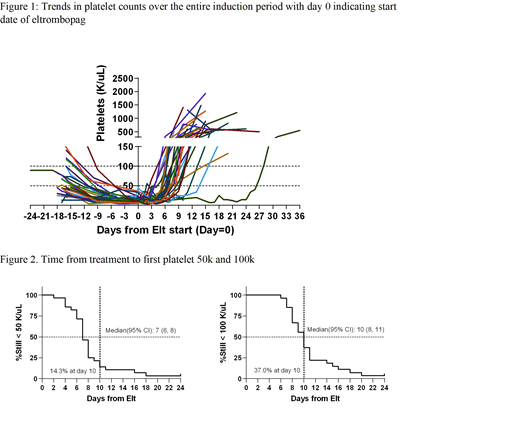
The median eltrombopag treatment duration was 10 days (range, 8-11 d), and no dose escalations were required. Trends in the levels of plt count over time are shown in Figure 1. Median times from the start date of eltrombopag to plt >50,000/µL, and >100,000/µL were 7 d (range, 6-8) and 10 d (range, 8-11), respectively (Figure 2). By day 24 of IC, 30 of 31pts (97%) had plt >50,000/µL. The median peak plt count reached on eltrombopag was 606,000/µL (range, 280- 1,935,000/µL). The median time to reach peak plt count from start date of eltrombopag was 14 d (range, 13-36). Pts received a median of 4 units of plt transfusions (range, 3-11) in the first 14 days of induction (1 unit every 3.6 days), while during the eltrombopag treatment period, the median requirement decreased to 2 units (range, 0-6) or 1 unit every 4.8 d (P=.04). Ninety percent of pts achieved complete remission at a median time of 30 d [95% confidence interval (CI), 25-34d] from IC.
No death occurred during treatment. The most common any grade toxicities (incidence > 15%) were maculopapular rash (28%), nausea (24%), increased alkaline phosphatase (24%), anorexia (20%), headache (20%), increased aspartate aminotransferase (16%), hypocalcemia (16%), and hypokalemia (16%). Grade 3/4 toxicities were reported at 4% (n=1) for febrile neutropenia, fever, pain, decreased white blood cell count, hypocalcemia, hypokalemia, generalized muscle weakness, maculopapular rash, and thromboembolic event. Median overall survival time from IC was 11.2 months (95% CI, 1.9, 14.8 m).
Conclusion: In this study, eltrombopag appeared to hasten plt recovery and reduce plt transfusions without increasing rates of refractory disease in older FLT3-negative AML pts undergoing IC, without any limiting toxicities. Additional analyses are currently underway to evaluate durability of platelet responses, responses in other cell lineages and survival.
Mukherjee:Bristol-Myers Squibb: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; McGraw Hill Hematology Oncology Board Review: Other: Editor; Pfizer: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Projects in Knowledge: Honoraria; Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Partnership for Health Analytic Research, LLC (PHAR, LLC): Consultancy. Hobbs:Amgen: Research Funding; SimulStat Inc.: Consultancy. Gerds:Celgene Corporation: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Sierra Oncology: Research Funding; Roche: Research Funding; Imago Biosciences: Research Funding; Pfizer: Consultancy; Incyte: Consultancy, Research Funding. Advani:Abbvie: Research Funding; Glycomimetics: Consultancy, Research Funding; Kite Pharmaceuticals: Consultancy; Amgen: Research Funding; Pfizer: Honoraria, Research Funding; Macrogenics: Research Funding. Nazha:Tolero, Karyopharma: Honoraria; MEI: Other: Data monitoring Committee; Novartis: Speakers Bureau; Jazz Pharmacutical: Research Funding; Incyte: Speakers Bureau; Daiichi Sankyo: Consultancy; Abbvie: Consultancy. Saunthararajah:EpiDestiny: Consultancy, Equity Ownership, Patents & Royalties; Novo Nordisk: Consultancy. Maciejewski:Novartis: Consultancy; Alexion: Consultancy. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal