Background: Severe deficiency of plasma ADAMTS13 activity resulting from autoantibodies against ADAMTS13 is the primary cause of immune thrombotic thrombocytopenic purpura (iTTP). The anti-ADAMTS13 IgGs may bind and inhibit plasma ADAMTS13 activity and/or accelerate its clearance from circulation. Approximately 40% of iTTP patients who survived the initial episode may experience disease recurrence (e.g. exacerbation and/or relapse). However, a reliable biomarker that predicts disease recurrence is still lacking. The present study sought to determine the roles of plasma ADAMTS13 activity, antigen, inhibitors, and anti-ADAMTS13 IgG at various stages of the disease process in predicting recurrence (e.g. exacerbation and/or relapse) in adult patients with iTTP.
Methods: 93 episodes from 83 unique iTTP patients who underwent therapeutic plasma exchange (TPE) therapy at UAB Medical center from April 2006 to December 2018 were enrolled into the study. Clinical and laboratory parameters were collected at diagnosis, and ADAMTS13 biomarkers (e.g. activity, inhibitors, antigen, and anti-ADAMTS13 IgG) were determined in samples collected on admission, 3-7 days following the first TPE, and at clinical response (prior to discharge). Various statistical analyses including Kaplan-Meier survival and Cox analyses were performed to assess the clinical relevance of each biomarker at various stages of the disease course.
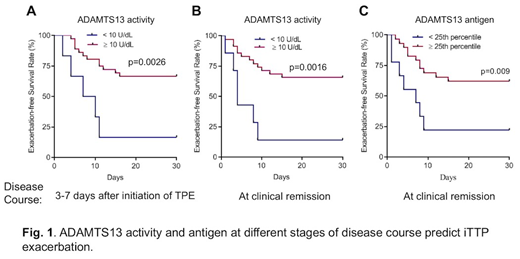
Results: We found that plasma ADAMTS13 activity and antigen on admission had no significant value in predicting iTTP exacerbation (disease recurrence within 30 days of discontinuation of TPE) or relapse (the disease recurrence after 30 days of discontinuation of TPE). However, high levels of anti-ADAMTS13 IgG on admission were associated with an increased risk of exacerbation (HR=2.1, 95% CI, 1.0-4.1, p=0.34) or recurrence within one year (HR=2.0, 95% CI 1.0-3.9, p=0.041). More interestingly, low plasma ADAMTS13 activity (<10 U/dL) 3 to 7 days after initiation of TPE (HR=3.8, 95% CI 1.3-11.0, p=0.015) or at clinical remission (median 7 days, IQC 6-10 days) when platelet counts and lactate dehydrogenase (LDH) levels had normalized (HR=4.3, 95% CI 1.6-11.8, p=0.004) and low levels of plasma ADAMTS13 antigen at clinical remission (HR=3.2, 95% CI 1.1-7.5), even when body mass index, race, and partial thromboplastin time (PTT) were all included as covariates for the analysis, were associated with the increased risks of iTTP recurrence.
Conclusions: Longitudinal assessment of plasma ADAMTS13 biomarkers (e.g., activity, antigen and anti-ADAMTS13 IgG) is of a critical value for predicting iTTP recurrence. These results suggest the need for additional therapeutic coverage (such as the use of rituximab, corticosteroids, and caplacizumab) to prevent iTTP recurrence after discharge from the hospital and supports the concept for achieving the biochemical remission (e.g. the normalization of ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG) for the long-term management of patients with iTTP.
Zheng:Shire/Takeda: Research Funding; Clotsolution: Other: Co-Founder; Ablynx/Sanofi: Consultancy, Speakers Bureau; Alexion: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal