Introduction:
An autologous CD3+ cell collection is the first step required for a CAR-T therapy. Up to now, in the commercial setting, one of the available models predicting the time or processed patient blood volume needed in order to gain the minimum-targeted absolute count of CD3+ cells relies on a formula provided by Novartis (Reference Model - RM), which assumes these parameters to be inversely proportional. We here aimed to develop a refined and more precise model, which enables to predict the needed processed blood volume in patients with low pre-apheresis peripheral CD3+ counts for gaining the targeted CD3+ cell count of e.g. 1.0x10⁹, using the patient's pre-apheresis peripheral CD3+ cell values, the collected absolute CD3+ cell counts, and the effectively processed blood volumes (Bern model - BM).
Methods:
We analyzed 16 lymphocyte apheresis procedures in adult patients (ALL: n=1; B-cell lymphomas: n=15) with a median age of 70.5 years (r 25 - 81 years) performed at the University Hospital Bern, Switzerland, for the production of Kymriah® (Novartis) in the period from December 2018 till July 2019. All cell collections were performed on the Spectra Optia (Terumo BCT©) device. Continuous Mononuclear Cell Collection (CMNC) procedure was used. We measured the peripheral CD3+ cell counts of the patients' blood and the collected products by means of multi-parameter flow cytometry (BD FACSCanto II). The inversely proportional function (RM) did not fit well our data, which was the trigger to look for an alternative function. Best fit of the series of data points and establishing of the function calculation has been performed by using Excel 2019 statistics, using power function modeling.
Results:
The median peripheral CD3+ cell level at start of apheresis was 1.29x10⁹/ml (r 0.11x10⁹/ml - 2.66x10⁹/ml). Low peripheral CD3+ counts (defined as < 0.7x10⁹/ml) were found in 5 out of 16 patients, whereas 11 patients showed normal peripheral CD3+ counts. Interestingly, in our hands lymphocyte collections at low pre-apheresis CD3+ counts was successful reaching the target >1.0x10⁹ CD3+ cells defined by the manufacturer. The median collection duration was 176 minutes (r 114 - 286 minutes) and the median ratio of processed blood volume to WBV (patient whole blood volume) was 3.1 (r 1.50 - 6.70). There was a significant negative correlation between CD3+ cell levels and blood volume processed/WBV (Spearman rank correlation, r: -0.92, p=0.0285). The median collected CD3+ cell number in the lymphocyte harvests was 6.43 x109 (r 1.88x109 - 11.90x109), fulfilling the required minimum of CD3+ cells in all 16 patients.
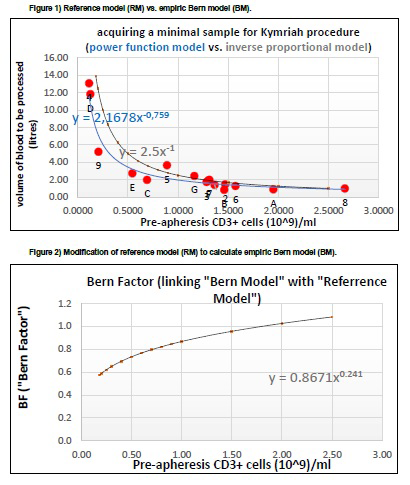
In Figure 1, each data point labeled by 1-9 or A- G represents an individual patient. The function of RM y=2.5x-1 (plotted in gray) is the intuitive, inverse proportional model provided by Novartis to calculate the minimum targeted cell count for production of Kymriah®. The function of BM y=2.167x-0,759 (plotted in blue) is our empiric model best fitting to our patients' data. Both models provide numerically similar results in case of normal pre-apheresis CD3+ cell counts, but differ significantly in the area of low pre-apheresis CD3+ values.
To illustrate all similarities and differences between BM and RM in a single plot, one can present this relation as follows: BM = 2.167(pCD3+)-0,759 is equal to 2.5(pCD3+) -1(RM) * 0.8671(pCD3+)0,241 where pCD3+ is the pre-apheresis CD3+ cell count expressed in 109 cells/ ml. Using this approach we obtain the following final formula: BM = RM * BF where BF (Bern Factor) is defined here as 0.8671 * (pCD3+)0.241. Figure 2 presents a graphic illustration of the formula for the BF. The function in Figure 2 is linking the RM and BM. For low CD3+ values, the BF is lower than for the normal CD3+ values.
Conclusion:
In the area of lower pre-apheresis CD3+ values, lymphocyte apheresis showed to be more efficient than expected by the manufacturer's model and the collection could be performed faster than assumed. According to our data the function y=2.167x-0,759 is more precise for calculating the needed processed blood volume for gaining the minimum required CD3+ cell count of 1.0x10⁹. Further investigations are needed to evaluate the applicability of the above Bern Model to predict processed blood volumes in patients with low peripheral CD3+ counts and to explain the phenomenon why the collection seems less efficient for patients with normal peripheral CD3+ cells.
Jalowiec:Pfizer: Other: Travel grant; Amgen: Other: Travel grant; Novartis: Other: Travel grant. Baerlocher:Novartis: Research Funding. Zeerleder:Alexion: Speakers Bureau; Jazz Pharma: Speakers Bureau; Sanofi/Genzyme: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal