Background:
Immune thrombocytopenia (ITP) is the most common autoimmune cytopenia diagnosed in children, typically causing a severely low platelet count, variable bleeding symptoms, and reductions in health-related quality of life (HRQoL). Eltrombopag is an established therapy for pediatric patients with chronic ITP. Favorable safety and efficacy were shown in the PETIT and PETIT2 trials leading to FDA-approval for children with chronic ITP in 2015. Off-label use of eltrombopag for adults with newly diagnosed ITP has been described in two small single-center trials. Gomez-Almaguer et al. reported 100% response (platelets >30 x 109/L) at completion of therapy and 66.7% relapse-free survival at 1 year in a single-arm study of dexamethasone in combination with 4 weeks of eltrombopag upfront in adult patients with newly diagnosed ITP, better outcomes than expected for comparable patients treated with steroids alone. In a second study, Tripathi et al. found 76% of steroid-nonresponsive patients had a durable response to eltrombopag after 3 months of therapy. Pediatric hematologists are already using thrombopoietin receptor agonists (TPO-RAs) in some cases of newly diagnosed ITP, according to a retrospective study by Neunert et al. TPO-RAs may be an efficacious first-line therapy for newly diagnosed ITP patients who require treatment.
Study Design and Methods:
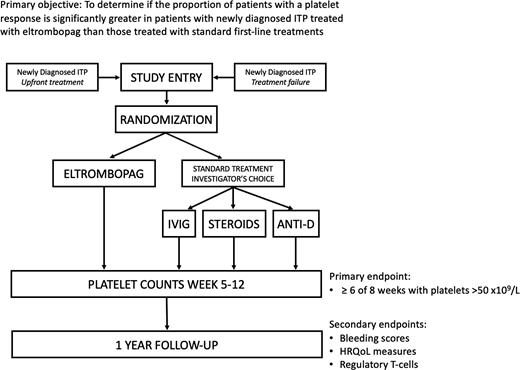
The PINES (Pediatric ITP Newly diagnosed patients Eltrombopag vs Standard therapy) Study, NCT03939637, is an investigator-initiated prospective, open label, randomized, multi-center trial sponsored by the ITP Consortium of North America (ICON) and funded by Novartis. The primary objective is to determine if the proportion of patients with a platelet response, defined as ≥ 6 of 8 weeks with platelets >50 x109/L during weeks 5-12 of therapy without rescue treatment, is significantly greater in patients with newly diagnosed ITP treated with eltrombopag than in those treated with standard first-line treatments. This is a primary outcome used in a previous pediatric study of eltrombopag in chronic ITP (PETIT2) and is a clinically relevant outcome measuring a sustained, rather than transient, platelet response. Patients (n=156) from 20 ICON centers will be randomized 2:1 to receive the experimental treatment, eltrombopag, or investigator's choice of 3 standard front-line therapies: prednisone, intravenous immune globulin, or anti-D globulin at protocol-specified doses (Figure).
Eligible patients are ages 1 to <18 with primary ITP, within 3 months of diagnosis, with platelet count <30 x109/L who require pharmacologic treatment from the perspective of the treating clinician. There are 2 treatment groups: 1) upfront treatment, defined as patients within 10 days of ITP diagnosis with no prior treatment, and 2) treatment failure, defined as patients previously managed with observation or a first-line standard agent. Patients are excluded if they have severe bleeding, defined by Buchanan Overall Grade 4 or 5 bleeding or bleeding requiring emergent treatment in the opinion of the provider.
Patients will be followed for 1 year. Patients may receive prednisone, intravenous immune globulin, or anti-D globulin as rescue treatments beyond their study-assigned treatment in the first 12 weeks of the study. Patients randomized to the eltrombopag arm may continue this treatment throughout the 1-year duration of study participation if needed, with guidelines given for dose adjustments. Treatment after the first 12 weeks of study in the standard therapy arm or for patients originally assigned to eltrombopag who do not respond is at the discretion of the treating physician.
Randomization is stratified by age and treatment status (upfront treatment vs. treatment failure). A one-sided z-test, at alpha=0.025, will be used to compare the proportion of patients who have a platelet response between the two arms. All randomized patients will be analyzed in the intention-to-treat analysis. Secondary objectives include comparison of bleeding scores (WHO Bleeding Scale and Modified Buchanan Score), changes in HRQoL (measured by the Kids ITP Tool, Hockenberry Fatigue Scale, PROMIS, and Global Change Scale), and changes in percentage of CD4+25+Foxp3+ regulatory T cells. Samples will be banked for optional future biology studies. Site activation and enrollment began in May 2019, and updated enrollment data will be presented at the meeting.
Shimano:Novartis: Research Funding; Pfizer: Research Funding; Daiichi Sankyo: Research Funding. Grace:Novartis: Research Funding; Agios Pharmaceuticals, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bennett:Novartis: Research Funding. Neufeld:Octapharma, Shire Pharmaceuticals (Baxalta), Novo Nordisk, Celgene, NHLBI/NIH: Research Funding; Octapharma, Agios, Acceleron, Grifols, Pfizer, CSL Behring, Shire Pharmaceuticals (Baxalta), Novo Nordisk, ApoPharma, Genentech, Novartis, Bayer Healthcare: Consultancy; Octapharma: Other: study investigator, NuProtect study (Octapharma-sponsored). London:United Therapeutics: Consultancy; ArQule, Inc: Consultancy. Despotovic:Novartis: Research Funding; Amgen: Research Funding; Dova: Honoraria.
Eltrombopag is a thrombopoietin receptor agonist FDA-approved for use in chronic ITP.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal