Background: Sickle cell disease (SCD) patients are at significant risk for stroke and silent cerebral infarcts. At least 33% of adults have cognitive dysfunction. However, access to specialized assessments is limited, and there is currently an unmet need for a fast, easy to administer, screening tool for cognitive impairment in SCD. The Rowland Universal Dementia Assessment Scale (RUDAS) is a 6-item task-based questionnaire that evaluates executive function, memory, language, visual-spatial function, praxis and judgment. It has been validated in many cultures and neurocognitive diseases other than SCD.
Hypothesis: Poor RUDAS performance is associated with the presence of SCD complications independent of age, socioeconomic and education factors.
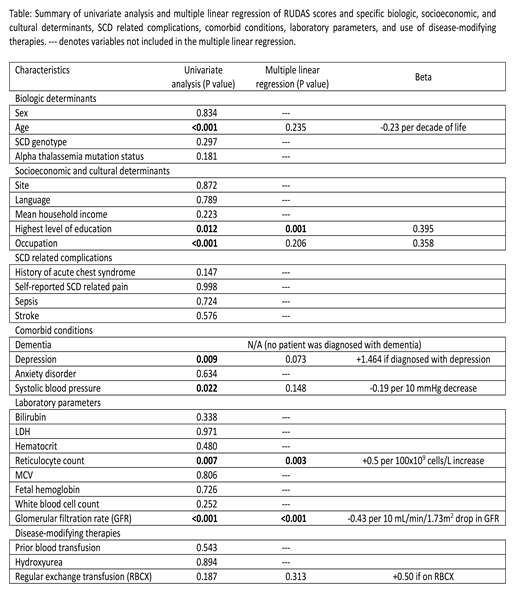
Methods: Study design: cross-sectional, two adult sickle cell comprehensive care centers in Canada. Inclusion criteria: out-patients ≥18 years-old; all SCD phenotypes. Exclusion criteria: inability to obtain informed consent and/or follow study instructions. Intervention: RUDAS was administered twice, 2-4 months apart, in French or English, based on the patient's preference. Survey on demographics and patient-reported outcomes (PROMIS® tools for Depression and Anxiety) were completed. Baseline characteristics, SCD complications, and laboratory results were collected. Statistical plan: t-tests, Fisher exact and chi-squared tests, for continuous and discrete variables respectively, were performed to identify possible association between RUDAS and biologic, socioeconomic, and cultural factors, SCD related complications, comorbid conditions, laboratory parameters, and use of disease-modifying therapy (Table). Associations with univariate P <0.05 were included in the multiple linear regression model. Multicollinearity was assessed.
Results: Of the 252 participants, 92 were from Centre Hospitalier de l'Université de Montréal in Montréal, 160 were from University Health Network in Toronto. Median age at time of survey was 31.5 years (IQR 25-44). Female to male ratio was 1.15. Sickle genotype was distributed as follows: SS 55% (N=138), SC 32% (N=80), other sickle genotypes 13% (N=34). Median RUDAS score was 26 (IQR 24-28), mean score ± standard deviation was 26.0±2.9. Suspected cognitive impairment (defined as RUDAS score <23/30) was found in 12% (N=29) of the participants. On univariate analysis, RUDAS score declined significantly with age (P<0.001), lower eGFR (P<0.001), lower systolic blood pressure (P=0.022), and lower reticulocyte count (P=0.007), while higher level of education (P=0.012), employment and/or active enrolment in a study program (P<0.001), and diagnosis of depression (P=0.009) were predictive of higher RUDAS scores (Table). Reticulocyte count, eGFR, and highest level of education remained independent predictors of RUDAS score on multiple linear regression (P=0.003, <0.001, and 0.001 respectively; see Table for effect size). Center, language of administration, age and diagnosis of depression were not associated with RUDAS score on multiple regression. R2 of the model was 0.323. All variance inflation factors in the model were <2.0.
Conclusions: Reticulocyte count and eGFR, but not SCD genotype, being independent predictors of RUDAS suggests disease phenotype may contribute to neurocognitive decline and deserves further exploration. RUDAS does not appear to be influenced by age, language of administration, socioeconomic status, and depression, on multiple regression with mild collinearity. Interestingly, education was independently associated with RUDAS score, despite previous studies showing RUDAS was not biased by education. Recruitment is ongoing at two additional sites to further delineate these relationships and to explore the role of silent cerebral infarct in neurocognitive decline in SCD patients. RUDAS may be a promising tool to identify the patients at higher risk for cognitive impairment who may benefit from access to specialized neurocognitive, educational and social interventions.
Kuo:Agios: Consultancy; Alexion: Consultancy, Honoraria; Apellis: Consultancy; Bioverativ: Other: Data Safety Monitoring Board; Bluebird Bio: Consultancy; Celgene: Consultancy; Novartis: Consultancy, Honoraria; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal