Background: Hydroxyurea (HU) has emerged as an important disease-modifying therapy for children and adults with sickle cell anemia (SCA), but has traditionally been underutilized. Consensus, evidence-based guidelines published by the National Heart, Lung, and Blood Institute (NHLBI) in 2014 recommended broadening the use of HU for SCA, but the impact of these recommendations on HU utilization is unknown. The objective of this abstract is to determine if HU utilization in children and adults with SCA living in Florida increased following publication of the 2014 Guidelines. We hypothesized that limitations in care coordination and implementation resulted in minimal increases in the rates of HU utilization. We further hypothesized that individuals living more than 45 minutes from a comprehensive sickle cell center would be less likely to be prescribed HU in the prior 12 months.
Methods: This study is a cross sectional analysis utilizing the OneFlorida Clinical Data Research Network (CDRN), which provides access to electronic health record and claims data for over 15.4 million patient records standardized to the PCORnet Common Data Model v4.1. Possible SCA cases were identified by International Classification of Diseases, 9th (ICD-9) and 10th (ICD-10) revision codes. Patients were eligible if they were at least 9 months of age and had two or more health encounters in which an ICD-9 or ICD-10 code for SCA was used. The primary endpoint was one or more HU prescriptions written or filled in a given calendar year between 2012 and 2017. In order to examine trends in HU utilization, segmented regression analyses of an interrupted time series were conducted. Logistic regression was performed to identify patient characteristics independently associated with HU utilization in 2017. Covariates of interest included age, gender, acute healthcare utilization (emergency department visits and hospitalizations), and distance to comprehensive sickle cell care (defined by zip code+4). Adjusted Odds Ratios (OR) and 95% confidence intervals (CI) were reported. Thirteen comprehensive sickle cell centers were identified based on local expert opinion, review of the American Society of Hematology Find a Hematologist database, and involvement in recent multicenter clinical trials.
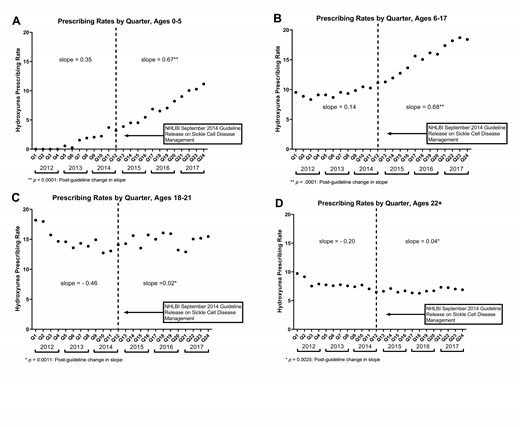
Results: 9,532 unique patients were identified with a mean age in 2017 of 21 years (SD 17.6). 57.4% were female, 76.2% were Black, 6% were Hispanic, 65.2% had three or more acute healthcare visits, 23% lived more than 45 minutes from a comprehensive sickle cell center, and approximately 73% were insured by Medicaid. Between 2012 and 2017, there was a 4.7% increase in HU utilization (12.7% vs. 17.4%, p<0.0001); see figure for trends in prescriptions written per quarter for each year stratified by age. Patients 6-17 years (OR 1.95, 95% CI 1.71-2.22, p<0.0001) and 18-21 years (OR 2.32, 95% CI 1.89-2.85, P<0.0001) were more likely to be prescribed HU compared to patients 22 years of age and older. Patients less than 6 years of age were less likely to be prescribed HU (0.81, 95% CI 0.68-0.97, p=0.02). Males (OR 1.47, 95% CI 1.31-1.65, p<0.0001) and individuals with three or more acute healthcare visits in a year (OR 22.56, 95% CI 17.03-29.89, p<0.0001) were more likely to be prescribed HU. No differences in HU utilization in 2017 were identified for individuals living 45 minutes or more from a comprehensive sickle cell center (OR 0.89, 95% CI 0.78-1.02, P=0.09).
Conclusions: These findings suggest there has been a slight, but statistically significant, increase in HU utilization in children and adults with SCA in Florida since publication of the 2014 NHLBI Guidelines. HU is being prescribed to more pediatric patients less than 18 years of age. However, HU remains drastically underutilized and appears to be preferentially prescribed to patients with three or more hospitalizations or emergency department visits per year. Additional research is needed to determine predictors of HU utilization and implementation strategies to improve prescribing rates. The OneFlorida CDRN provides an excellent resource to track quality metrics for SCA in Florida.
Black:Micelle BioPharma: Research Funding; Prolong Pharmaceuticals: Consultancy; Sanofi: Consultancy; Sancilio and Company: Research Funding; NHLBI: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; HRSA: Research Funding.
Hydroxyurea for children less than 2 years of age
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal