
Introduction: Elderly pts with R/R AML not eligible for cytotoxic therapy have limited therapeutic options, and dismal outcomes with available therapies. In preclinical studies, inhibition of BCL-2 and MDM2 with Ven and Idasa, respectively, has demonstrated potent synergistic apoptotic activity. In this ongoing, open-label, Phase Ib study, the safety, tolerability, and preliminary efficacy of Ven+cobimetinib (Arm A) and Ven+Idasa (Arm B) is being assessed in R/R AML (NCT02670044). Initial analysis indicated a tolerable safety profile for Ven+Idasa. Here, we present updated safety and efficacy results from Arm B.
Methods: Pts (≥60 years) with R/R AML or secondary AML, previously treated for an antecedent hematologic disease but treatment naïve for AML, and ineligible for cytotoxic therapy/allogeneic stem cell transplant were enrolled. The maximum tolerated dose of Ven+Idasa was determined by two-dimensional dose escalation. Pts received Ven orally (PO) daily (400 or 600mg) + Idasa PO daily on Days (D) 1-5 (150mg, 200mg, or 400mg) in 28-day cycles. Responses were assessed according to revised International Working Group Response Criteria 2003. Pharmacokinetic (PK) analyses were performed on plasma samples on Cycles (C) 1 and 2, D1 and 5, and C4, D1. Exploratory assessments included minimal residual disease (MRD), assayed centrally at Covance Laboratories using 8-color flow cytometry. Data cut-off was June 21, 2019.
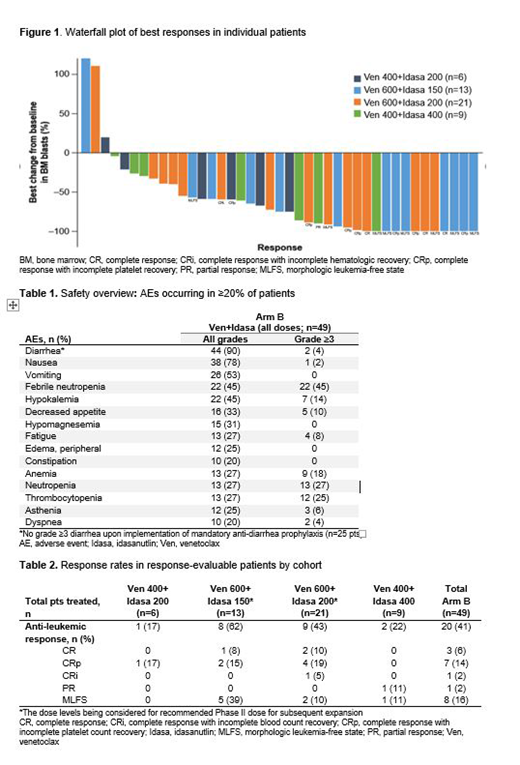
Results: At data cut-off, 49 pts were treated with Ven+Idasa. Median age was 72 years (range 62-93); Eastern Cooperative Oncology Group performance status 0-1: 84%; refractory AML: 57%; relapsed AML: 33%; and secondary previously untreated AML: 10%; Intermediate-I or Intermediate-II European Leukemia Net (ELN) risk classification: 66%; adverse ELN classification: 30%; de novo (49%) versus secondary (51%) AML; and median prior lines of treatment: 1 (range 1-4). Most common adverse events (AEs; any grade) irrespective of attribution were diarrhea (90%) and nausea (78%); the most common grade ≥3 AEs were febrile neutropenia (45%), neutropenia (27%), and thrombocytopenia (25%; Table 1). Laboratory tumor lysis syndrome occurred in 3 pts; none resulted in treatment discontinuation. Ven and Idasa treatment discontinuation due to AEs were noted in 18% and 20%, respectively, most commonly due to infections. 30- and 60-day mortality rates were 6% and 17%, respectively. No apparent PK drug-drug interaction was found between Ven and Idasa; overlap in Ven and Idasa exposure was substantial over the doses tested. Anti-leukemic response rate (complete response [CR] + CR with incomplete platelet count recovery [CRp] + CR with incomplete blood count recovery [CRi] + partial response [PR] + morphologic leukemia-free state [MLFS]) across all dose levels was 41% (Table 2). Across the two Ven 600mg cohorts being considered for the recommended Phase II dose (RP2D), the anti-leukemic response rate was 50% (CR+CRp+CRi rate 29%). Median time to CR+CRp+CRi+PR was 1.4 months (range 1-3), with a median response (CR+CRp+CRi) duration of 4.9 months (range 0.6-9.7). Median overall survival in all pts and in the Ven 600mg cohorts was 4.4 months and 5.7 months, respectively, with a median follow-up of 3.4 months (range 0.03-18). Individual pt responses are shown in Figure 1. MRD negativity (<0.1%) was achieved in 45% (5/11) of pts with CR+CRp+CRi. Updated pre- and post-therapy mutation and BCL-2 family protein data and association with clinical response will be presented.
Conclusions: The non-chemotherapy combination of Ven+Idasa demonstrated encouraging safety and efficacy in elderly pts with R/R AML who were ineligible for cytotoxic chemotherapy. The anti-leukemic response rate at the dose levels being considered for the RP2D was 50%, with a CR+CRp+CRi rate of 29%. Updated predictive biomarker data will be presented. Evaluation of Ven+Idasa RP2D is ongoing, and will be followed by dose expansion.
Daver:Otsuka: Consultancy; NOHLA: Research Funding; Jazz: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy; BMS: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Forty-Seven: Consultancy; Novartis: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Consultancy; Pfizer: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Genentech: Consultancy, Research Funding; Glycomimetics: Research Funding; Servier: Research Funding; Incyte: Consultancy, Research Funding. Garcia:Abbvie: Research Funding; Genentech: Research Funding. Jonas:AbbVie, Amgen, Celgene, GlycoMimetics, Jazz, Pharmacyclics, Tolero: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Accelerated Medical Diagnostics, AROG, Celgene, Daiichi Sankyo, Esanex, Forma, Genentech/Roche, GlycoMimetics, Incyte, LP Therapeutics, Pharmacyclics: Research Funding; AbbVie, Amgen, GlycoMimetics: Other: Travel expenses. Kelly:Novartis, Bayer, Janssen, Pharmacyclics, Celgene, Astrazeneca, Seattle Genetics: Honoraria, Speakers Bureau; Takeda: Research Funding; Genentech, Verastem: Consultancy. Assouline:Janssen: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Brandwein:Celgene: Consultancy, Honoraria, Research Funding; Jazz Pharma: Consultancy, Honoraria; Otsuka: Honoraria; Roche: Research Funding; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding. Fenaux:Celgene Corporation: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Aprea: Research Funding. Olin:Ignyta: Research Funding; Clovis: Research Funding; Spectrum: Research Funding; Revolution Medicine: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria; Pfizer: Research Funding; Genentech: Consultancy, Research Funding; Daiichi Sankyo: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Mirati Therapeutics: Research Funding; MedImmune: Research Funding; AstraZeneca: Research Funding. Martinelli:Amgen: Consultancy, Other: trial grant; Ariad: Consultancy, Other: trial grant; Incyte: Consultancy, Other: trial grant; Pfizer: Consultancy, Other: trial grant; Roche: Consultancy, Other: trial grant; Celgene: Consultancy, Honoraria, Other: trial grant; Janssen: Consultancy, Other: trial grant; Abbvie: Consultancy, Honoraria, Other: trial grant; Novartis: Consultancy, Other: trial grant; Daiichi Sankyo: Consultancy, Honoraria. Pigneux:Novartis: Honoraria; Roche: Honoraria; Pfizer: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria; Astellas: Honoraria; Daichi: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; Amgen: Honoraria. Pollyea:Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Diachii Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Powell:Rafael Pharmaceuticals: Consultancy, Research Funding; Janssen: Research Funding; Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding. Roboz:Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Tafuri:Celgene: Research Funding; Novartis: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding. Vey:Novartis: Consultancy, Honoraria; Janssen: Honoraria. Yee:Agensys, Astex, Hoffman La Roche, MedImmune, Merck, Millenium, Roche/Genentech: Research Funding; Novartis, Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas, Celgene, Otsuka, Shire, Takeda: Membership on an entity's Board of Directors or advisory committees. Dail:Genentech: Employment, Equity Ownership. Green:Genentech Inc.: Employment. Kirschbrown:Genentech, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Hong:Roche: Equity Ownership; Genentech Inc.: Employment, Equity Ownership. Ott:Roche: Employment, Equity Ownership. Onishi:Genentech, Inc.: Employment. Wang:Genentech, Inc.: Employment; Roche: Equity Ownership. Konopleva:Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding. Andreeff:Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; NIH/NCI: Research Funding; CPRIT: Research Funding; Breast Cancer Research Foundation: Research Funding; Oncolyze: Equity Ownership; Oncoceutics: Equity Ownership; Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership; Aptose: Equity Ownership; BiolineRx: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Amgen: Consultancy; AstaZeneca: Consultancy; 6 Dimensions Capital: Consultancy; Reata: Equity Ownership.
Venetoclax (VEN; ABT-199/GDC-0199) is a highly selective, potent, oral B-cell lymphoma-2 (BCL-2) inhibitor. Idasanutlin (idasa; RG7388) is an orally available, small molecule antagonist of MDM2 (mouse double minute; Mdm2 p53 binding protein homolog).
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal