Background: In sickle cell disease (SCD), hemoglobin S (HbS) polymerizes upon deoxygenation, reducing red blood cell (RBC) deformability. RBC deformability can be measured over a range of oxygen concentrations using a Laser Optical Rotational Red Cell Analyzer (Lorrca) ektacytometer (RR Mechatronics, Zwaag, the Netherlands) with Oxygenscan module. The Oxygenscan measures 3 key parameters: 1) EImax, RBC deformability at normoxia; 2) EImin, RBC deformability upon deoxygenation; and 3) the point of sickling (PoS): the oxygen tension at which a 5% decrease in normoxic EI is observed during deoxygenation, reflecting the patient-specific pO2 at which sickling begins (Figure 1A). Previously we showed that Oxygenscan parameters correlate with measures of SCD disease severity and hemolysis (absolute reticulocyte count, %fetal hemoglobin, %HbS, total Hb, %dense RBC, (Rab et al. Blood 2018). In this study, we investigated the relationship between oxygenscan parameters and incidence of vaso-occlusive crisis (VOC), and we tested the ability of the Oxygenscan parameters to assess response to hydroxyurea (HU) and chronic transfusion (CTF) in patients with SCD.
Methods: We analyzed 2 cohorts: a European cohort (EUC) of 62 SCD patients (all HbSS or HbS/β-thalassemia), enrolled at either University Medical Center Utrecht (UMCU, n= 42) or Hospital Lyon (LIBM, n=20), and a US cohort (USC) of 97 SCD patients enrolled at Texas Children's Hospital (TCH). Differences in Oxygenscan parameters in SCD patients without/with VOC (requiring a doctor's evaluation) in the past 2 years were measured in 46 adult EUC SCD patients and 80 pediatric USC SCD patients. EUC patients without VOC (n=18, median age 40.6 years (y); 11 female (F), 44% on HU, 6% on CTF, 28% on both treatments), were compared to patients with a positive history of VOC (n=28, median age 23.9y, 13F, 39% on HU, 11% on CTF and 11% on both). Patients without VOC in the USC (n= 34, median age 8.0y, 15F, 53% on HU, 15% on CTF and 21% on both treatments) were compared to patients with VOC (n=46, median age 11.8y, 20F, 74% on HU, 13% on CTF and 11% on both treatments). To establish treatment related Oxygenscan parameter changes, we analyzed RBCs from 9 SCD patients from UMCU (median age 19y, 5F), before and during HU treatment (measurements performed at baseline, and 1, 3 and 6 months after starting HU), 7 SCD patients from UMCU (median age 26.7y; 6F) before and after transfusion and 17 SCD patients from TCH (median age 10.8y; 6F) on HU before and after transfusion.
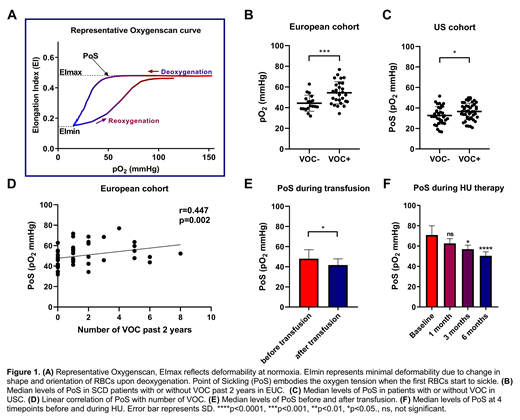
Results: In the EUC, PoS differed significantly between patients without VOC in the last 2 years (median 41.6mmHg) and patients with VOC in the last 2 years (median 53.7 mmHg, p<0.001, Figure 1B). In the USC, PoS was also lower in patients without VOC compared to those with a VOC in past 2 years (p<0.05, Figure 1C). EImin in both cohorts was significantly lower in patients who experienced VOC (p<0.05). EImax did not show a significant difference in both cohorts. Correlation of PoS with VOC episodes was significant in the EUC (r=0.447, p<0.01, Figure 1D) and USC (r=0.228, p<0.05), indicating that RBCs start to sickle at a higher pO2 in patients where VOC occur more often. Differences found in median PoS between EUC and USC could be due to differences in treatment (EUC 30% no treatment, USC 5% no treatment), difference in age (EUC median age 28.5y, USC median age 11.6y), genetic background or technical differences between Oxygenscan devices.
Transfusion improved EImax, EImin, and PoS (USC: p<0.001, p<0.0001, and p<0.01, EUC: all parameters p<0.05 Figure 1E). HU treatment improved all parameters after 3 and 6 months compared to baseline (p<0.05 and p<0.0001 Figure 1F).
Conclusion: Our results show that RBC from SCD patients without VOC in the last two years were able to tolerate lower oxygen concentrations before sickling (PoS). RBCs from patients without VOC were also more deformable when deoxygenated (EImin) compared to patients who had experienced one or more VOCs in the last two years. In contrast, RBC deformability, when oxygenated (EImax) was not different in patients with or without VOC in the last two years. All 3 Oxygenscan parameters significantly improved with standard of care SCD treatments, namely CTF and/or HU. We therefore conclude that the PoS, EImax and EImin are useful biomarkers of clinical severity and treatment response, and may be essential in monitoring novel SCD treatments as part of a clinical trial as a surrogate endpoint.
Rab:RR Mechatronics: Research Funding. van Wijk:Agios Pharmaceuticals: Consultancy, Research Funding; RR Mechatronics: Research Funding. Bos:RR Mechatronics: Research Funding. Nur:Novartis Pharmaceuticals: Consultancy. Cnossen:NWO: Other: Governmental grants , ZonMW, Innovation fund and Nationale Wetenschapsagenda 2018; Roche: Other: Travel Grants; Takeda: Other: Travel Grants, Research Funding; Shire: Other: Travel Grants, Research Funding; Baxter: Other: Travel Grants, Research Funding; Sobi: Research Funding; CSL Behring: Other: Travel Grants, Research Funding; Nordic Pharma: Research Funding; Novo Nordisk: Research Funding; Bayer: Other: Travel Grants, Research Funding; Pfizer: Other: Travel Grants, Research Funding. van Beers:Agios Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal