Rate of Sickle Hemoglobin Recovery in Sickle Cell Disease Patients Undergoing Red Blood Cell (RBC) Exchange Transfusion is Associated with Age of Patients and Number of RBC Units Transfused
Introduction: Automated and manual red blood cell exchange (RBCX) transfusions are useful in the primary and secondary prevention of sickle cell disease (SCD) complications (Ware et al., 2012). The ability to consistently maintain sickle hemoglobin (HbS) below target (30% or 50% depending on indication) is quite variable (Kuo et al., 2012). With emerging indications such as silent cerebral infarction, it is imperative that effective means of chronic transfusion to maintain appropriate hematological and clinical targets be identified. We hypothesize the rate of HbS recovery is dependent on the individual's hemolytic and erythropoietic rate. The purpose of this study is to evaluate the effect of the rate of erythropoiesis and hemolysis on HbS recovery in SCD patients undergoing RBCX.
Methods: Fifteen (15) patients were prospectively recruited from the adult SCD transfusion program (9 automated, 6 partial manual), from December 2018 to July 2019, and followed through one exchange cycle (4 weeks). Automated and partial manual exchange transfusion protocols have been previously described elsewhere (Canadian Haemoglobinopathy Association Consensus Statement on the Care of Patients with Sickle Cell Disease in Canada, Version 2.0, Ottawa; 2015). Exclusion criteria included active hydroxyurea or erythropoietic stimulating agents use, reported ill health in the preceding 4 weeks, co-morbid hemolytic condition or non-HbSS genotype. Hemoglobin, hematocrit, HbS, lactate dehydrogenase (LDH), reticulocyte count, indirect bilirubin, and serum erythropoietin level were determined for each patient: pre- and post- first exchange, weekly for 3 weeks and pre- second exchange (the 4th week). Descriptive variables were either expressed as means ± SD or median (IQR), based on normality, while linear regression was performed for continuous variables. Co-variates were included in multivariable analysis if P < 0.10. Multivariable linear regression was conducted to examine the potential association between the change in HbS over one RBCX cycle and age of patients, pre-RBCX hematocrit, LDH, and number of RBC units transfused.
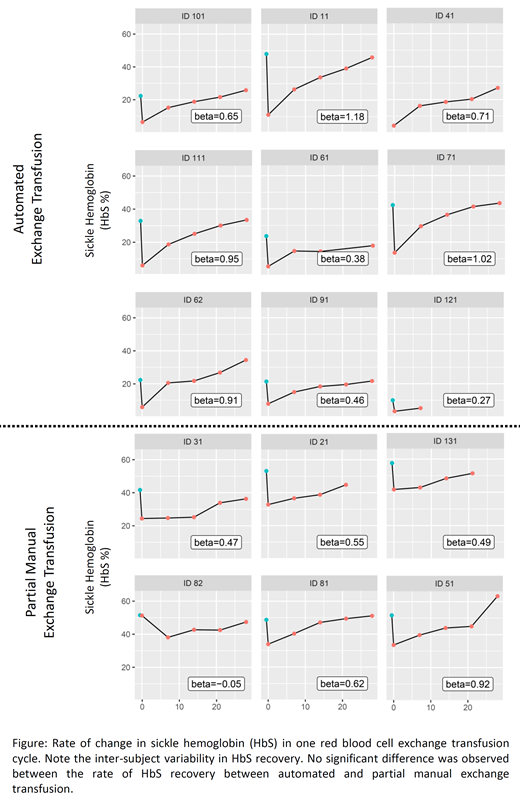
Results: We identified 36 eligible patients from the Program database, after which 15 consented to participate in the study. Mean age was 32.9 ± 12.3 years, consisting of 7 males and 8 females. There was an association between the rate of change in HbS and age of patients (p=0.035), pre-RBCX hematocrit (p=0.030) and number of transfused RBC units (p=0.030). LDH showed a trend towards reduced rate of change in HbS (p=0.069). Rate of change in HbS was not associated with automated vs. partial RBCX (Figure), female vs. male patients, pre-RBCX HbS, erythropoietin, indirect bilirubin, reticulocyte and age of transfused RBCs. Age of patients (p<0.001) and number of units transfused (p=0.010) were independently associated with the rate of change of HbS, after adjusting for hematocrit and LDH. For every decade increase in age, the rate of HbS recovery was 3% lower in one RBCX cycle. For each additional unit of RBC exchanged, the rate of HbS recovery was 0.78% higher in one RBCX cycle.
Conclusion: This is the first study to evaluate the determinants of variability in the rate of HbS recovery in SCD patients on RBCX. Age of patients and number of RBC units transfused may underlie the significant variability in achieving HbS targets in SCD patients on RBCX. We failed to show a significant difference in the rate of change in HbS between automated and partial manual exchange transfusion. Reduction in HbS recovery with advancing age may be related to erythropoietic reserve in the patients' marrow. However, the influence of units of RBC recovery appeared paradoxical, and the finding may be spurious due to small sample size. It may therefore be possible to appropriately titrate the number of RBC units with advancing age of SCD patients with a view to achieving desired HbS targets.
Patriquin:Ra Pharma: Consultancy, Research Funding; Apellis: Consultancy, Honoraria, Research Funding; Alexion: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding. Kuo:Agios: Consultancy; Alexion: Consultancy, Honoraria; Apellis: Consultancy; Bioverativ: Other: Data Safety Monitoring Board; Bluebird Bio: Consultancy; Celgene: Consultancy; Novartis: Consultancy, Honoraria; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal