
Background
CD19-targeted chimeric antigen receptor-engineered (CD19 CAR)-T cell immunotherapy has shown promising efficacy in patients with relapsed or refractory (R/R) B-cell malignancies. The potential benefits of repeat infusions of CD19 CAR-T cells are unknown, and the factors associated with response, CAR-T cell in vivo expansion, and progression-free survival (PFS) after repeat infusion of CD19 CAR-T cells have not been investigated.
Methods
We analyzed the outcomes of patients with R/R B-cell malignancies after a second infusion of CD19 CAR-T cells (CART2) on a phase 1/2 trial (NCT01865617) at our institution. Responses after CAR-T cell therapy were evaluated around day 28 after infusion and defined according to the 2018 NCCN guidelines for acute lymphoblastic leukemia (ALL), 2018 iwCLL for chronic lymphocytic leukemia (CLL), and the Lugano criteria for non-Hodgkin lymphoma (NHL). Logistic, Cox and linear regression were used for multivariable analyses of response, progression-free survival and peak CD8+ CAR-T in blood, respectively. Bayesian model averaging was performed for variable selection.
Results
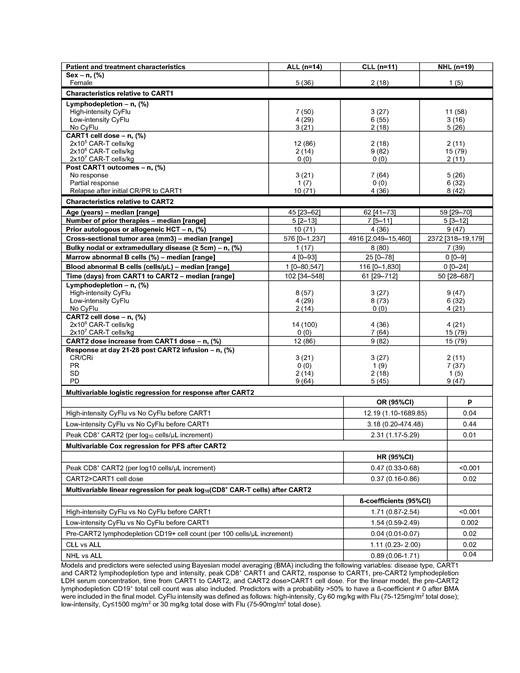
Forty-four patients evaluable for response (ALL, n=14; CLL, n=11; NHL, n=19) were included in this study. The median age at the time of CART2 was 58 (range, 23-73). Patients were heavily pre-treated (median prior therapies, 6; range, 2-13), and 16 patients (36%) had bulky (≥ 5cm) nodal or extramedullary disease. The median time from the first CAR-T infusion (CART1) to CART2 was 70 days (range, 28-712). Twenty-eight patients (64%) had received a CART1 dose ≥ 2x106 CAR-T cells/kg. Fifteen patients (32%) had not responded to CART1, 22 (50%) relapsed or progressed after having initially responded (complete response [CR], n=15; partial response [PR], n=7) to CART1; 7 (16%) received CART2 in PR after CART1. All characteristics are shown in the Table.
We observed responses in all disease types, including 3 of 14 ALL patients (21%; all CR/CRi), 4 of 11 CLL patients (36%; CR/CRi, n=3; partial response [PR], n=1), and 9 of 19 NHL patients (47%; CR, n=2; PR, n=7). After a median follow-up of 43 months (range, 16-66) in alive and responding patients, the estimated 4-year PFS probability in responders was 23% (95% CI, 9-59%). The 4-year overall survival probability in responders was 36% (95% CI 19-71%) compared to 24% (95% CI, 12-47) in non-responders.
Multivariable logistic regression modeling identified predictors of response after CART2: CART1 lymphodepletion (high-intensity cyclophosphamide and fludarabine [CyFlu] vs no CyFlu, OR=12.19, 95% CI, 1.10-1689.85, p=0.04), and peak of in vivo CAR-T cell expansion after CART2 (OR=2.31 per log10 CD8+ CAR-T cell/µL increase, 95% CI, 1.17-5.29, p=0.01).
In a multivariable Cox model, a higher peak of CD8+ CAR-T cells after CART2 (HR=0.47 per log10 CD8+ CAR-T cell/µL increase, 95%CI, 0.33-0.68, p<0.001); CART2 > CART1 cell dose was associated with longer PFS (HR=0.36, 95% CI, 0.16-0.86, p=0.02). This suggested that CD8+ CAR-T cell peak after CART2 and factors increasing CART2 peak (e.g. prevention of immune rejection or increase in the infused cell dose) are key elements associated with outcomes of CART2. Hence, we looked at factors associated with higher CD8+ CART2 peak. In multivariable linear regression, CART1 CyFlu predicted a higher peak of CD8+ CAR-T cells after CART2 (high-intensity CyFlu vs no CyFlu, p<0.001 ; low-intensity CyFlu versus no CyFlu, p=0.002) after adjusting for disease type (CLL vs ALL, p=0.02; NHL vs ALL, p=0.04) and the total CD19+ cell count in blood (p=0.02).
CyFlu being the most commonly used lymphodepletion prior to CAR-T cell therapy, we evaluated the impact of CART1 CyFlu lymphodepletion intensity by comparing high-intensity to low-intensity CyFlu in our multivariable models. Logistic regression suggested higher probabilities of response to CART2 in patients who received high-intensity compared to low-intensity CyFlu prior to CART1 (OR=3.83, 95%CI, 0.85-21.83, p=0.08). In multivariable analysis, CART1 high-intensity CyFlu was associated with higher CD8+ CAR-T cell numbers at day 60 after CART2 compared to low-intensity CyFlu (p=0.01) after adjusting for disease type and total CD19+ cell count in the blood.
Conclusion
Our findings suggest outcomes after second infusions of CD19 CAR-T cells might be improved with high-intensity CyFlu lymphodepletion prior to CART1 and by increasing the CAR-T cell dose at the time of CART2.
Hirayama:DAVA Oncology: Honoraria. Till:Mustang Bio: Patents & Royalties, Research Funding. Kiem:Rocket Pharma: Consultancy, Equity Ownership; Homology Medicines: Consultancy, Equity Ownership; CSL Behring: Consultancy; Magenta Therapeutics: Consultancy. Shadman:Mustang Biopharma: Research Funding; Gilead: Research Funding; Bigene: Research Funding; AstraZeneca: Consultancy; Merck: Research Funding; Atara: Consultancy; AbbVIe: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; TG Therapeutics: Research Funding; Sound Biologics: Consultancy; Pharmacyclics: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Verastem: Consultancy; Celgene: Research Funding; Acerta: Research Funding; Emergent: Research Funding; Sunesis: Research Funding. Cassaday:Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Kite/Gilead: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Other: Spouse's disclosure: employment, stock and other ownership interests. Riddell:Juno Therapeutics: Equity Ownership, Patents & Royalties, Research Funding; Adaptive Biotechnologies: Consultancy; Lyell Immunopharma: Equity Ownership, Patents & Royalties, Research Funding. Maloney:BioLine RX, Gilead,Genentech,Novartis: Honoraria; Juno Therapeutics: Honoraria, Patents & Royalties: patients pending , Research Funding; Celgene,Kite Pharma: Honoraria, Research Funding; A2 Biotherapeutics: Honoraria, Other: Stock options . Turtle:Precision Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Patents & Royalties: Co-inventor with staff from Juno Therapeutics; pending, Research Funding; Caribou Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Other: Ad hoc advisory board member; Allogene: Other: Ad hoc advisory board member; Nektar Therapeutics: Other: Ad hoc advisory board member, Research Funding; T-CURX: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Ad hoc advisory board member; Humanigen: Other: Ad hoc advisory board member.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal