Background: Systemic Lupus Erythematosus (SLE) is a chronic inflammatory autoimmune disorder with multi-organ involvement, including skin rash, joint pain, neurological dysfunction, pulmonary fibrosis, vasculitis, and renal failure. Previously it has been reported that SLE patients have a lower percentage of regulatory T cells (Tregs) and when compared to healthy population, Tregs derived from SLE patients show defect in their suppressor function. Our group at MD Anderson Cancer Center has already shown that a significantly lower dose of cord blood (CB) Tregs as compared to conventional T cells (Tcon) in the donor graft is able to prevent graft vs host disease (GVHD). Furthermore, adoptive therapy with CB Tregs is being explored as a therapy for bone marrow failure including aplastic anemia as a single agent in the non-transplant setting. Therefore, we hypothesized that adoptive therapy with CB Tregs may be utilized for the treatment of SLE.
Method: For examining the efficacy of CB Tregs in vivo, we developed a humanized SLE model, where female Rag2-/-γc-/- mice were transplanted with 3 ~ 4 x 106 human SLE-PBMCs by intravenous injection on day 0. The mice were allowed to develop disease (control) and at 1 weeks post-transplant a single dose of 1x107 CB Tregs was injected through the tail vein (treatment). Mice peripheral blood (PB) was assessed weekly for their cell compartment composition by using flow cytometry; double stranded DNA (dsDNA IgG) and plasma cytokines. Mice were monitored twice per week for weight loss, GVHD score and survival. Weekly urine collection was performed to analyze for albumin and creatinine. At the time of euthanasia, harvested organs were analyzed by flow cytometry, and immunohistochemistry.
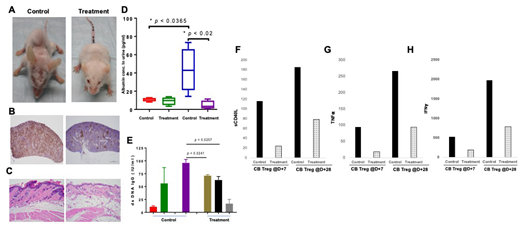
Result: Single injection of CB Tregs was sufficient to slow down the phenotype of SLE as shown in figure 1A, where the physical appearance of CB Treg treated mice was significantly better than the control and it correlated with a significantly lesser CD3+ infiltrates in the spleen of the treatment vs. control mice (Figure 1B) and a similar finding was observed in the CD20 infiltrate in the renal tissue (data not shown). While a widespread parakeratosis in the skin of the control mice, almost complete resolution was observed in the treatment arm (figure 1C). In addition, a lack of lymphoid infiltrate in the kidney and resolution of splenic lymphoid hyperplasia was observed in the CB Treg recipients (data not shown). In another experiment, we studied the role of weekly injection of 1x107 CB Tregs with the first injection administered at week 4 after the lupus inoculation. Significant improvement was observed in the urine albumin (p<0.02) (Fig 1D) as well as urine creatinine (p<0.0006) levels; which correlated with the significant improvement in the dsDNA IgG levels in the treatment arm compared to control (Fig 1E), respectively. We also measure the durability of single dose CB Treg injection, where a decrease in the 5-week plasma inflammatory cytokines including sCD40L (Fig 1F); TNFα (Fig 1G) and IFNγ (Fig 1H) was observed irrespective of CB Treg injection on day+7 or day+28 after the lupus PBMC injection.
Conclusion: We conclude that adoptive therapy with CB Tregs is a viable option for SLE and additional studies are planned to optimize the dose and schedule details.
Khoury:Angle: Research Funding; Stemline Therapeutics: Research Funding; Kiromic: Research Funding. Iyer:Seattle Genetics, Inc.: Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Genentech/Roche: Research Funding; Incyte: Research Funding; Arog: Research Funding. Parmar:Cellenkos Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal