Cancer immunotherapies have resulted in a paradigm shift in therapy. One of the most successful approaches has been administration of antibodies targeting immune checkpoint inhibitors (ICI), such as programmed death-1 (PD-1), that can reinvigorate functionally exhausted T cells. Unfortunately, durable tumor regression is limited to a minority of patients, and relapse remains a significant concern. Combining novel immunotherapies with ICI s a promising strategy to bolster antitumor responses and response rates.
Natural killer (NK) cells mediate direct tumor cell lysis, effectively target MHC low or null transformed cells and are key regulators of T cell responses through the production of inflammatory cytokines and chemokines. In many cancers, NK cell numbers are low, and their functional responses are sub-optimal. The use of allogeneic peripheral blood NK cells for immunotherapy has shown significant clinical promise for the treatment of various cancers. However, sourcing NK cells for adoptive cell therapy has been limited by both cell number and quality. Thus, we developed a robust manufacturing system for the differentiation and expansion of high-quality NK cells derived from induced pluripotent stem cells (iPSCs) that can be combined with ICI antibodies for multiple tumor types.
To interrogate the ability of iPSC-derived NK cells to synergize with ICI therapy, we developed an in vitro 3D tumor spheroid system to model the combinatorial effects of activated CD3+ T cells, iPSC-derived NK (iNK) cells and ICI blockade for anti-tumor function in a more physiological context than the standard 2D cultures and in real time. Using SKOV-3 (an ovarian cancer line) spheroids as targets in a 160-hour killing assay, we found that iNK cells could mediate significant, but not complete destruction of tumor spheroids (46% tumor reduction). Twice as many activated CD3+ T cells by themselves also induced significant but incomplete tumor spheroid destruction (58% tumor reduction). Combined iNK and activated CD3+ T cells led to robust target cell destruction (71% tumor reduction). Importantly, adding anti-PD-1 antibody (mAb) to activated CD3+ T cells and iNK cells led to near complete elimination of tumor spheroid targets, with >99% tumor reduction. Cytokine and chemokine secretion analyses in co-cultures of activated CD3+ T cells and iNK cells revealed synergistic production of CCL3 and CCL4 for T cell recruitment, and TNF and IFN-γ to augment anti-tumor responses.
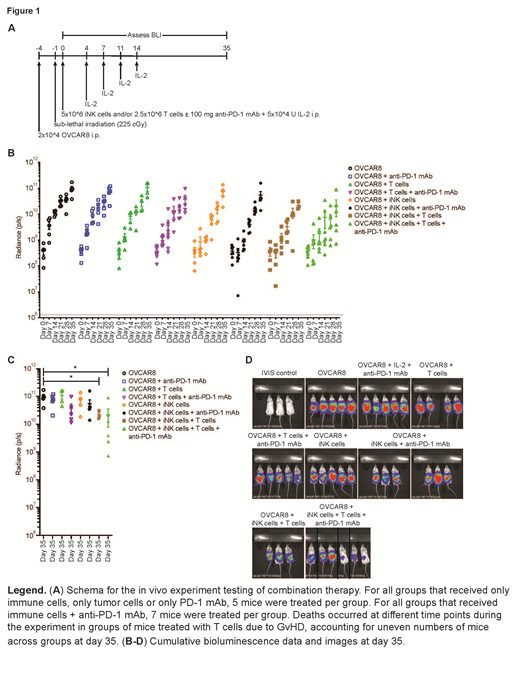
To determine whether these striking 3D tumor spheroid results simulate the in vivo setting, we injected luciferase-expressing OVCAR8 (a human ovarian cancer line) cells into the peritoneal cavities of immunodeficient NSG mice. Following sublethal irradiation, we treated groups of mice with either anti-PD-1 mAb, activated CD3+ T cells or iNK cells alone or in various combinations. IL-2 was injected i.p. twice weekly for two weeks into all mice except those in the tumor alone group (Figure 1A). In mice that received iNK cells alone, significant tumor control was observed over the first 21 days but was not sustained. Tumor control was similar between groups of mice that received iNK cells and mice that received iNK cells and anti-PD-1 mAb. Mice treated with either anti-PD-1 mAb or activated CD3+ T cells alone exhibited similar rates of tumor growth relative to the untreated group. Modest tumor control was observed in the group of mice that received combined activated CD3+ T cells and anti-PD-1 mAb, though the effect did not reach statistical significance. Durable tumor control past day 21 was observed in groups of mice that received either combined activated CD3+ T cells and iNK cells or activated CD3+ T cells + iNK cells with anti-PD-1 mAb (Figure 1B). Importantly, 50% of mice in the activated CD3+ T cells + iNK cells + anti-PD-1 mAb group exhibited tumor bioluminescence readings well below other treatment group at day 35 (Figure 1C, D). Collectively, these data demonstrate that iNK cells can serve as an off-the-shelf source of high-quality NK cells and synergize with anti-PD-1 ICI therapy to enhance anti-tumor T cell responses in vitro and in vivo, providing a novel immunotherapeutic platform for tumors in which iNK cells, activated CD3+ T cells or anti-PD-1 mAb therapy alone is not sufficiently effective. The current program is under clinical investigation and can be found at clinicaltrials.gov NCT03841110.
Miller:Moderna: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics, Inc: Consultancy, Research Funding; CytoSen: Membership on an entity's Board of Directors or advisory committees; OnKImmune: Membership on an entity's Board of Directors or advisory committees; Dr. Reddys Laboratory: Membership on an entity's Board of Directors or advisory committees; GT BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bjordahl:Fate Therapeutics, Inc.: Employment. Gaidarova:Fate Therapeutics, Inc: Employment. Mahmood:Fate Therapeutics, Inc: Employment. Rogers:Fate Therapeutics, Inc: Employment. Moyar:Fate Therapeutics, Inc: Employment. Blazar:Abbvie Inc: Research Funding; KidsFirst Fund: Research Funding; Childrens' Cancer Research Fund: Research Funding; Leukemia and Lymphoma Society: Research Funding; Regeneron Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics and BlueRock Therapeuetics: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics, Inc.: Research Funding; Tmunity: Other: Co-Founder; BlueRock Therapeutics: Membership on an entity's Board of Directors or advisory committees; RXi Pharmaceuticals: Research Funding; Alpine Immune Sciences, Inc.: Research Funding; Kamon Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees; Five Prime Therapeutics Inc: Co-Founder, Membership on an entity's Board of Directors or advisory committees. Kaufman:FATE Therapeutics: Consultancy, Research Funding. Valamehr:Fate Therapeutics, Inc: Employment. Cichocki:Fate Therapeutics, Inc: Research Funding.
NK cells
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal