
Background: Venetoclax (Ven) is a highly selective, potent, oral BCL-2 inhibitor that induces apoptosis in multiple myeloma (MM) cells and has shown synergistic activity with the proteasome inhibitor (PI) bortezomib (B) and dexamethasone (d). Ven ± d had encouraging clinical efficacy in both t(11;14) MM and in pts irrespective of genetic background when administered with B, with a tolerable safety profile in Phase 1 studies. Here, we provide updated efficacy and safety of Ven vs placebo (Pbo) + Bd in pts with relapsed/refractory (RR) MM, including subgroup analyses, in the BELLINI study.
Methods: BELLINI (NCT02755597) was a Phase 3, randomized, double-blind, multicenter study of Ven or Pbo + Bd in pts with RRMM who received 1 - 3 prior therapies and were either sensitive or naïve to PIs. Pts were randomized 2:1 to receive Ven 800 mg/day or Pbo + Bd. Cycles 1-8 were 21-day with B 1.3 mg/m2 on Days 1, 4, 8, 11 + d 20 mg on Days 1, 2, 4, 5, 8, 9, 11, 12. Cycles 9+ were 35-day with B 1.3 mg/m2 on Days 1, 8, 15, 22 + d 20 mg Day 1, 2, 8, 9, 15, 16, 22, 23. The primary endpoint was progression-free survival (PFS) by independent review committee (IRC).
Results: A total of 291 pts were randomized, 194 to the Ven arm and 97 to the Pbo arm. Median age was 66 (range, 36 - 87); 53% had ISS II/III disease; 54% received 2 or 3 prior lines of therapy; 59% had prior stem cell transplant; 70% had prior PI, 68% had prior immunomodulatory drug, 41% had both. Among pts with evaluable results, 18% had high-risk cytogenetics, 13% had MM positive for t(11;14), and 79% had high levels of BCL-2 protein by immunohistochemistry (IHC).
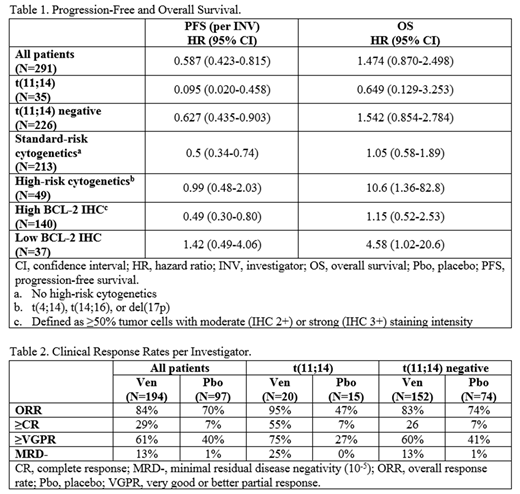
In the primary endpoint analysis per IRC, the median PFS was 22.4 months (m) in Ven vs 11.5 m in Pbo (HR=0.630, p=0.01), with a median follow-up of 18.7 m (as of 26 Nov 2018). As of updated analysis based on a data cut-off of 18 March 2019, the median PFS (per investigator [INV]) was 22.9 m in Ven vs 11.4 m in Pbo (HR=0.587, p=0.001; Table 1), with a median follow-up of 22.7 m. Per INV, higher overall response (ORR, 84% vs 70%, p=0.009) and very good partial or better response (≥VGPR, 61% vs 40%, p<0.001; Table 2) rates were observed in Ven vs Pbo. Minimal residual disease negativity rate (by next-generation sequencing) was also higher in the Ven arm vs Pbo (MRD- [10-5], 13% vs 1%). Median duration of response was 23.4 m for Ven and 12.8 m for Pbo. In the overall population, median overall survival (OS) was not reached in either arm but continued to favor Pbo (HR 1.474, 95% CI=0.870-2.498). A total of 70 deaths have been reported, 51 (26%) in the Ven arm and 19 (20%) in the Pbo arm.
In the safety population (N=289), the most common treatment-emergent adverse events (TEAEs; Ven/Pbo) were diarrhea (59%/48%), nausea (37%/22%), constipation (35%/31%), and fatigue (31%/32%). The most common Grade 3/4 TEAEs were neutropenia (18%/8%), pneumonia (17%/12%), anemia (16%/15%), thrombocytopenia (15%/30%), and diarrhea (15%/12%); 23%/12% discontinued Ven due to a TEAE. The rates of serious AEs (51%/51%) and serious infections (30%/28%) were comparable between arms. There were 69 deaths in the safety population: in the Ven arm, 14 were treatment-emergent (TE; treatment start to 30 days after discontinuation) and 36 were non-TE (>30 days after treatment discontinuation); in the Pbo arm, 1 was TE and 18 were non-TE.
In the t(11;14) subgroup, median PFS has not been reached for pts receiving Ven, but was 9.3 m for Pbo (HR=0.095; per INV). In the t(11;14)-negative (neg) subgroup, median PFS was 22.4 m and 10.7 m for Ven and Pbo, respectively (HR=0.627; per INV). Median OS has not been reached in either arm for the t(11;14) and t(11;14)-neg subgroups, although the HR favored Ven in t(11;14) pts, and Pbo in t(11;14)-neg pts. Analyses indicate that low BCL-2 expression by IHC and high-risk cytogenetics (defined as t(4;14, t(14;16), or del(17p)) were associated with decreased PFS and OS in the Ven arm (Table 1). In the high-risk cytogenetics pts, median PFS was 11.4 m in both arms (HR=0.99), and median OS has not been reached in either arm but favors Pbo (HR=10.6). In the subgroup with low BCL-2 expression by IHC, median PFS was 11.7 m and 17.0 m for Ven and Pbo, respectively (HR=1.42), and median OS was 21.3 m in the Ven arm and not reached in Pbo (HR=4.58).
Conclusions: Updated analysis of BELLINI continue to reflect a favorable benefit-risk profile in t(11;14) pts, with meaningful clinical responses and improvement in PFS, as well as a positive trend in OS in this subgroup when treated with Ven + Bd.
Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Harrison:Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cavo:sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria. De La Rubia:Janssen: Consultancy; Takeda: Consultancy; Celgene Corporation: Consultancy; AMGEN: Consultancy; AbbVie: Consultancy. Popat:Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses; Janssen: Honoraria, Other: travel support to meetings; Takeda: Honoraria, Other: travel, accommodations, expenses; GSK: Consultancy, Honoraria; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gasparetto:Celgene: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; BMS: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; Janssen: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed . Hungria:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salwender:Amgen: Honoraria, Other: Travel or accommodations; Takeda: Honoraria, Other: Travel or accommodations; Bristol-Myers Squibb: Honoraria, Other: Travel or accommodations; Sanofi: Honoraria, Other: Travel or accommodations; Celgene: Honoraria, Other: Travel or accommodations; AbbVie: Honoraria; Janssen Cilag: Honoraria, Other: Travel or accommodations. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Gay:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Mikala:Takeda: Honoraria; Roche: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Research Funding; Amgen: Honoraria; AbbVie: Honoraria, Research Funding. Punnoose:Genentech, Inc.: Employment; Roche: Other: Stock/stock options. Hong:Genentech Inc.: Employment, Equity Ownership; Roche: Equity Ownership. Sood:AbbVie: Employment, Other: Stock/stock options. Jalaluddin:AbbVie: Employment, Other: Stock/stock options. Ross:AbbVie: Employment, Other: Stock/stock options. Ward:AbbVie: Employment, Other: Stock/stock options. Maciag:AbbVie: Employment, Other: Stock/stock options. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding.
Venetoclax is a BCL-2 inhibitor that is FDA-approved in some indications. This presentation will focus on venetoclax for treatment of multiple myeloma, which is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal