
Introduction: Patients (pts) with RRMM have a poor prognosis, with limited treatment options and poor overall survival (OS). Despite the increasing use of lenalidomide (len) in frontline treatment of multiple myeloma, len-refractory pts are often excluded from randomized studies. In the few studies including len-refractory pts, median progression-free survival (PFS) of <11 months has been reported with carfilzomib (K)/dexamethasone (d), daratumumab (DARA)/bortezomib (V)/d, pomalidomide (pom)/Vd, or elotuzumab/pom-d treatment. Therefore, there is an unmet need for effective therapies to treat pts with len-refractory RRMM.
DARA is a human IgGκ monoclonal antibody targeting CD38 approved as monotherapy in RRMM and in combination with standard-of-care regimens for newly diagnosed multiple myeloma and RRMM. K is a proteasome inhibitor approved as monotherapy and in combination with len and d for RRMM. Here, with a median follow-up of 23.7 months, we present the final analysis of the treatment arm from a phase 1b study that evaluated DARA in combination with K and d (D-Kd) in pts with RRMM, including len-refractory pts.
Methods: MMY1001 (NCT01998971) is a multi-arm, phase 1b study evaluating DARA in combination with various backbone therapies. Eligible pts with RRMM in the D-Kd arm had an ECOG score of ≤2, were treated with >1 prior line of therapy, including V and an immunomodulatory drug, and were K naïve. All pts (N = 85) received 28-day cycles of D-Kd until disease progression. DARA was given weekly for Cycles 1-2, every 2 weeks for Cycles 3-6, and every 4 weeks thereafter. 10 pts received the first DARA dose as a single infusion (16 mg/kg Cycle 1 Day 1) and 75 pts received a split first dose (8 mg/kg Cycle 1 Days 1 and 2). Pts received K weekly on Days 1, 8, and 15 of each cycle (20 mg/m2 initial dose, escalated to 70 mg/m2 thereafter), and d (40 mg) weekly. The primary endpoint was safety and tolerability. Overall response rate was a secondary endpoint, and minimal residual disease (MRD) was an exploratory endpoint.
Results: Pts received a median of 2 (range, 1-4) prior lines of therapy. All pts received prior V; 81 (95%) received prior len (51 [60%] were len-refractory), and 13 (15%) received prior pom. 50 (59%) pts discontinued study treatment: 36 (42%) due to progressive disease, 6 (7%) due to patient withdrawal, 5 (6%) due to adverse event (AE), 2 (2%) due to physician decision, and 1 (1%) due to death.
In the safety-analysis set (N = 85), the most common (>10%) grade 3/4 treatment-emergent AEs were thrombocytopenia (32%), lymphopenia (25%), anemia (21%), neutropenia (21%), hypertension (20%), and asthenia (15%). Serious AEs occured in 41 (48%) pts; 9%, 19%, and 14% were related to DARA, K, and d, respectively. Median left ventricular ejection fraction did not notably change over time from baseline (64% at baseline [n = 84], 62% at Cycle 6 [n = 54], 61% at Cycle 12 [n = 47], 59% at Cycle 18 [n = 22], and 63% at Cycle 24 [n = 10]). 7 (8%) pts experienced grade 3/4 cardiac AEs of interest, 2 (2%) pts each with cardiac failure and sinus tachycardia, and 1 (1%) patient each with acute pulmonary edema, left ventricular failure, atrial fibrillation, and myocardial ischemia. Infusion-related reactions occured in 6 of 10 (60%) and 31 of 75 (41%) pts receiving single- and split-first doses of DARA, respectively; the vast majority were mild (grade 1/2) and occurred during the first infusion.
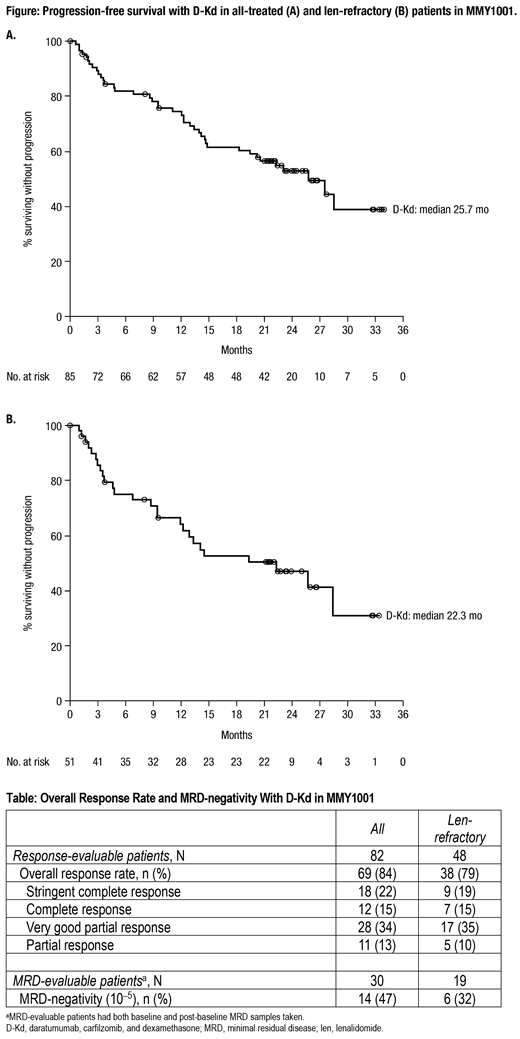
After a median follow-up of 23.7 (range, 0.5-34.7) months, median PFS was 25.7 months (95% confidence interval [CI], 14.8- not estimable [NE]; Figure) in all-treated and 22.3 months (95% CI, 12.0-NE) in len-refractory pts. The 24-month PFS rate was 53% in all-treated and 47% in len-refractory pts. Response and MRD-negativity rates are summarized in the Table. The median duration of response among pts that responded to treatment was 27.5 months (95% CI, 20.5-NE). A total of 32 (38%) pts received subsequent anticancer therapy, with a median time to subsequent therapy of 29.2 (95% CI, 19.5-NE) months. Median PFS on next line of therapy (PFS2) and OS were NE; the 24-month PFS2 rate was 61% (95% CI, 49-71) and the 24-month OS rate was 71% (95% CI, 60-80).
The DARA pharmacokinetic profile was consistent with previous studies, and no pts were positive for anti-DARA antibodies.
Conclusions: D-Kd was well tolerated, with no new safety concerns identified. Patients treated with D-Kd demonstrated deep responses and encouraging PFS outcomes, including in patients refractory to len.
Chari:Janssen, Celgene, Novartis Pharmaceuticals, Amgen, Bristol Myers Squibb, Pharmacyclics, Karyopharm, Sanofi, Seattle Genetics, OncoPeptides, Millenium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lonial:Celgene Corporation: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Amgen: Consultancy; BMS: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy; Karyopharm: Consultancy; Genentech: Consultancy. Martinez-Lopez:F. Hoffmann-La Roche Ltd: Honoraria; Janssen: Honoraria, Other: Advisory boards and Non-Financial Support ; Celgene: Honoraria, Other: Advisory boards and Non-Financial Support ; VIVIA Biotech: Honoraria; BMS: Honoraria, Other: Advisory boards; Incyte: Honoraria, Other: Advisory boards; Novartis: Honoraria, Other: Advisory boards; Amgen: Honoraria, Other: Non-Financial Support . Mateos:Pharmamar: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; EDO: Membership on an entity's Board of Directors or advisory committees. Bladé:Irctures: Honoraria; Janssen, Celgene, Amgen, Takeda: Membership on an entity's Board of Directors or advisory committees. Oriol:Celgene, Amgen, Takeda, Jansse: Consultancy, Speakers Bureau. Rodriguez Otero:Kite Pharma: Consultancy; Takeda: Consultancy; BMS: Honoraria; Celgene Corporation: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria. Jakubowiak:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Honoraria; SkyLineDx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria; KaryoPharm Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Olyslager:Janssen: Employment, Equity Ownership. Wang:Janssen: Employment. Nnane:Janssen: Employment, Equity Ownership. Ukropec:Janssen: Employment, Equity Ownership. Shreeve:Johnson & Johnson: Equity Ownership; Janssen: Employment. Qi:Janssen: Employment. Moreau:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
In the submitted abstract, we present data from a phase 1b clinical trial of a combination regimen that is not currently approved for the treatment of relapsed/refractory multiple myeloma. Components of the combination regimen including daratumumab and carfilzomib are, however, approved as monotherapy or in combination with standard-of-care regimens for treatment of patients with relapsed/refractory multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal