Background BCMA CAR-T cells have demonstrated substantial clinical activity against relapsed/refractory multiple myeloma (RRMM). In different clinical trials, the overall response rate (ORR) varied from 50% to 100%. Complete remission (CR) rate varied from 20% to 80%. Here we developed a BCMA CAR-T cell product manufactured via lentiviral vector-mediated transduction of activated T cells to express a second-generation CAR with 4-1BB costimulatory domain and evaluated the efficacy and safety, moreover, dynamics of immune cell subsets using single-cell mass cytometry during treatment were analyzed.
Methods Our trial (ChiCTR1800017404) is a phase 1, single-arm, open-label single center study to evaluate the safety and efficacy of autologous BCMA CAR-T treatment for RRMM. Patients were subjected to a lymphodepleting regimen with Flu and Cy prior to CAR-T infusion. BCMA CAR-T cells were administered as a single infusion at a median dose of 3.5 (1 to 6) ×106/kg. MM response assessment was conducted according to the International Uniform Response Criteria. Cytokine-release syndrome (CRS) was graded as Lee DW et al described (Blood.2014;124(2):188-195). Phenotypic analysis of peripheral blood mononuclear cells (PBMCs), frozen BCMA CAR-T aliquots, phenotype and in vivo kinetics of immune cell subsets after CAR-T infusion were performed by single-cell mass cytometry.
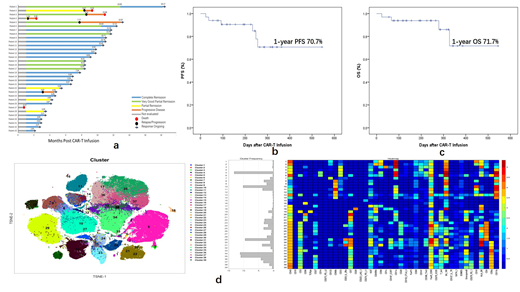
Results As of the data cut-off date (August 1st, 2019), 33 patients, median age 62.5 (49 to 75) years old were infused with BCMA CAR-T cells. The median observation period is 8.0 (0.7 to 18) months. ORR was 100% (The patient who died of infection at 20 days after CAR-T infusion were excluded). All the 32 patients achieved MRD negative in bone marrow by flow cytometry in 2 weeks after CAR-T infusion. Partial response (4 PR, 12.1%), VGPR (7 VGPR, 21.2%), and complete response (21 CR, 63.6%) within 12 weeks post CAR-T infusion were achieved. Durable responses from 4 weeks towards the data cut-off date were found in 28/33 patients (84.8%) (Figure 1a). All patients had detectable CAR-T expansion by flow cytometry from Day 3 post CAR-T cell infusion. The peak CAR-T cell expansion in CD3+ lymphocytes of peripheral blood (PB) varied from 35% to 95% with a median percentage of 82.9%. CRS was reported in all the 33 patients, including 4 with Grade 1, 13 with Grade 2 and 16 with Grade 3. During follow-up, 1-year progression-free survival (PFS) was 70.7% (Figure 1b) and overall survival (OS) was 71.7% (Figure 1c). Multivariate analysis of patients with PR and patients with CR+VGPR revealed that factors including extramedullary infiltration, age>60 years old, high-risk cytogenetics, late stage and CAR-T cell dose were not associated with clinical response (P>0.05). Single-cell mass cytometry revealed that the frequency of total T cells, CD8+ T cells, NK cells and CD3+CD56+ NKT cells in PB was not associated with BCM CAR-T expansion or clinical response. CD8+ Granzyme B+ Ki-67+ CAR-T cells expanded prominently in CRS period. As serum cytokines increased during CRS, non-CAR-T immune cell subsets including PD1+ NK cells, CD8+ Ki-67+ ICOS+ T cells expanded dominantly implying that non-CAR-T cells were also activated after CAR-T treatment. After CRS, stem cell like memory CAR-T cells (CD45RO+ CCR7- CD28- CD95+) remain the main subtype of CAR-T cells (Figure 1d).
Conclusions Our data showed BCMA CAR-T treatment is safe with prominent efficacy which can overcome the traditional high-risk factors. We also observed high expansion level and long-term persistence of BCMA CAR-T cells contribute to potent anti-myeloma activity. Stem cell like memory CAR-T cells might be associated with long-term persistence of BCMA CAR-T cells. These initial data provide strong evidence to support the further development of this anti-myeloma cellular immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal