INTRODUCTION: Daratumumab has been recently introduced in the treatment of patients with multiple myeloma (MM). Two phase II trials of this drug in relapsed/refractory AL amyloidosis were recently completed, but final reports have not been published so far. One randomized phase III trial in treatment-naïve patients with this diseases has recently completed enrollment and the results are eagerly awaited. However, several retrospective series showed encouraging response rates with daratumumab alone or in combination with bortezomib or immune modulatory drugs in AL amyloidosis. After this drug was marketed for MM in Italy, it became routinely accessible also to patients with myeloma-associated AL amyloidosis (MM-AL). We evaluated the efficacy of daratumumab in a consecutive series of patients with MM-AL in three centers of the Italian Amyloidosis Study Group.
METHODS: Consecutive patients with a diagnosis of MM-AL treated with daratumumab were included in the study. Patients received daratumumab 16 mg/Kg body weight e.v. once a week for the first two months and then every other week for the next four months and subsequently every 28 days. The standard DVd (daratumumab, bortezomib and dexamethasone) and DRd (daratumumab, lenalidomide and dexamethasone) regimens were also used in subsets of patients. Hematologic and organ responses were assessed according to the International Society of Amyloidosis (ISA) criteria.
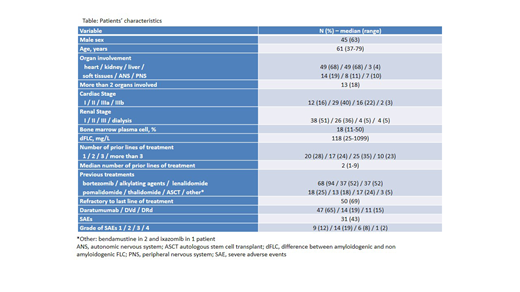
RESULTS: Seventy-two patients were included in the study (61 in Pavia, 10 in Padua and 1 in Turin), Table. Median age was 61 years (range: 37-79 years) and 45 (63%) patients were males. The heart and the kidney were involved in 49 patients each (68%). Median bone marrow plasma cell infiltrate was 18% (range: 11-50%). Median time from diagnosis to treatment initiation was 32 months (range: 3-185 months). Fifty (69%) patients were refractory to the line of therapy immediately preceding daratumumab. Median number of prior treatments was 2 (range: 1-9) and daratumumab was used as second line option in 20 (28%) cases. Forty-seven (65%) patients received daratumumab as single agent. The remaining patients received DVd 14 (19%) and DRd in 11 (15%) cases.
After 8 infusions 59 (82%) patients achieved a hematologic response (HR): complete response (CR) in 12 (16%), very good partial response (VGPR) in 30 (42%) and partial response (PR) in 17 (24%). Cardiac response was observed in 9 of 37 evaluable patients (24%) and renal response in 21 of 38 patients (55%). Among patients in VGPR, 4 did not qualify for CR due to the persistence of an abnormal free light chain (FLC) ratio (FLCR) despite normalization of involved FLC (iFLC) due to lower than normal levels of uninvolved FLC, while serum and urine immunofixation were negative.
After 16 infusions there was only a modest improvement of overall HR rate (60 patients, 83%), but quality of response improved: with CR in 22 patients (30%), VGPR in 21 (29%), and PR in 17 (24%). Of the 4 patients in iFLC-response after 8 infusions, 2 qualified for CR with normalization of FLCR after 16 infusions. In the other 2 patients FLCR did not normalize and we found persistence of the amyloid light chain at high-resolution immunofixation of serum and/or urine. No difference in HR was seen between patients treated with daratumumab single agent and those who received daratumumab combinations (after 16 infusions 81% vs. 88%, P=0.470). After 16 infusions, cardiac response was observed in 11 of 37 evaluable patients (29%) and renal response in 23 of 38 patients (60%). After a median follow-up of living patients after daratumumab initiation of 12 months, 5 subjects died due to disease progression and projected survival at 1 year was 95%.
CONCLUSION: Daratumumab is effective in heavily pretreated patients with MM associated AL amyloidosis. Quality of response improved over time with 60% of patients achieving CR or VGPR after 16 infusions. Suppression of uninvolved FLC may result in delayed normalization of FLC ratio in some patients.
Milani:Pfizef: Honoraria; Janssen: Honoraria. Larocca:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria. Oliva:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Petrucci:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Palladini:Celgene: Other: Travel grant; Janssen-Cilag: Honoraria; Sebia: Honoraria; Janssen-Cilag: Other: Travel grant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal