BACKGROUND
Real-world data on the use of pomalidomide (POM) for the treatment (Tx) of relapsed/refractory multiple myeloma (RRMM) are limited. The MIROIR study was designed to evaluate POM Tx in routine clinical practice in France. Here, we present results from a prespecified 4-year interim analysis.
METHODS
MIROIR is a multicenter, observational, ambispective, non-interventional study of POM in routine clinical practice. Adult patients (pts) with MM who initiated POM Tx in France between October 1, 2014, and September 30, 2018, were included. All pts were required to be enrolled in the French IMNOVID® registry. Data were collected from medical records of consenting pts. Key exclusion criteria included previous treatment with POM or simultaneous participation in a clinical trial. The primary endpoint is progression-free survival (PFS) at 6 months. Key secondary endpoints include time to next Tx (TTNT), overall survival (OS), and safety. This study is ongoing; targeted enrollment is 3000 pts (ClinicalTrials.gov, NCT02902900).
RESULTS
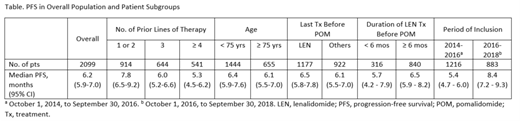
A total of 2099 pts were included in this analysis (median follow-up: 23.3 months; data cutoff: February 1, 2019). Median age was 70.0 years, and 655 pts (31.2%) were aged ≥ 75 years; 1134 pts (54.0%) were male. Median time from start of first-line Tx to POM initiation was 51.4 months. Pts had received a median of 3 prior lines of therapy (range: 0-9), with 914 (43.5%), 644 (30.7%), 312 (14.9%) and 229 pts (10.9%) receiving ≤ 2, 3, 4, and ≥ 5 prior lines, respectively. From 2014 to 2016, the median number of prior lines of therapy before POM initiation was 3, and from 2016 to 2018, the median was 2. Nearly all pts received prior lenalidomide (LEN; 97.0%) and bortezomib (96.7%).
POM was initiated at 4 mg/day in 1635 pts (77.9%) overall and in 1216 pts (84.2%) aged < 75 years and in 419 pts (64.0%) aged ≥ 75 years. Dexamethasone was prescribed at 20 mg/day and 40 mg/day in 507 (35.1%) and 732 pts (50.7%) aged < 75 years and in 405 (61.8%) and 62 pts (9.5%) aged ≥ 75 years.
Overall, the 6-month PFS rate was 51.7% (95% CI, 49.4%-54.1%). Other key PFS data in pt subgroups are reported in the Table. In the overall population, median TTNT, 12-month OS rate, and median OS were 10.4 months (95% CI, 9.7-11.2), 70.6% (95% CI, 68.5-72.6), and 24.6 months (95% CI, 22.9-not reached), respectively. Among 1164 pts (55.5%) with ≥ 1 adverse event (AE), the most common AEs were neutropenia (290 pts; 24.9%), infections (263 pts; 22.6%), thrombocytopenia (99 pts; 8.5%), and asthenia (87 pts; 7.5%). POM dose was reduced due to an AE in 20.7% of pts; POM Tx was interrupted or discontinued due to an AE in 36.2% and 15.2% of pts, respectively.
CONCLUSIONS
The results of this interim analysis confirm the efficacy of POM reported in clinical trials and underscore its role in Tx of RRMM, including after LEN Tx. Median PFS in pts with ≤ 2 prior Tx lines was numerically longer than in pts who had more Tx lines, supporting earlier Tx with POM. PFS outcomes were similar regardless of the duration of LEN Tx (< or ≥ 6 months) before initiation of POM and whether pts had received LEN or another Tx as their most recent therapy. The latter finding suggests that POM can be used after relapse or resistance to LEN and that there is no need to replace an IMiD agent with another class of treatment.
Decaux:Celgene Corporation, Janssen, Takeda, Amgen: Honoraria. Macro:Celgene, Janssen, Amgen, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support. Gourgou:Celgene: Employment, Equity Ownership. Lachenal:Celgene: Other: Scientific Comittee's. Stoppa:Celgene: Honoraria. Jaccard:Abbvie: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria. Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Perrot:jannsen: Honoraria, Membership on an entity's Board of Directors or advisory committees; takeda: Honoraria; Amgen: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding. Karlin:AMGEN: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Fohrer:Celgene: Consultancy, Honoraria. Leleu:Carsgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Hulin:celgene: Consultancy, Honoraria; Janssen, AbbVie, Celgene, Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal