BACKGROUND: Proteasome inhibitors (PIs) combined with an Rd backbone constitute commonly used regimens in RRMM in the US. Lack of data from head-to-head clinical trials comparing the efficacy of these PI-Rd triplets and the extent to which efficacy translates to benefit in routine care highlight the need for real-world evidence. We conducted a comparative effectiveness analysis of time to next therapy (TTNT) of these PI-Rd triplet combinations in a real-world representative cohort of RRMM patients.
METHODS: In this retrospective cohort study, RRMM patients who initiated an index regimen with IRd, KRd, or VRd in ≥2nd line of therapy (LOT 2+) were followed between 1/2014-9/2018 in Optum's deidentified national electronic health records database. This database captures information on all patients treated by >140,000 providers across 50 states in the US. It is representative of the US general population. Baseline data included: a modified frailty score utilizing age and Charlson Comorbidity Index1, ECOG performance status, CRAB symptoms, ISS stage, cytogenetic risk (high: del[17p], t[4;14], or t[14;16]), history of: cardiovascular disease, peripheral neuropathy, stem-cell transplant, and uncontrolled hypertension, prior PI and/or IMID exposure and refractory status, time from diagnosis to start of index LOT, time from start of frontline therapy to start of LOT 2, and treatment-free interval prior to index LOT. Duration of therapy (DOT) and TTNT were estimated using Kaplan-Meier methods and compared using stratified (by LOT) ± baseline-adjusted Cox proportional hazard models with a repeated measures analysis of pt-LOTs with robust sandwich estimators to account for inclusion of patients in multiple LOTs. The main analysis was repeated in subgroups a priori defined by frailty score, cytogenetic risk, prior PI and/or IMID exposure and LOT. Observations were censored at time of loss to follow up/end of study period (9/30/2018).
RESULTS: Overall, 650 patients with 733 pt-LOTs (IRd, n=168; KRd, n=208; VRd, n=357) were included; 20% of the RRMM patients in this cohort were treated at academic centers. In the I-/K-/V-Rd groups, respectively, median age was 71/65/71 years and 42/22/38% patients were ≥75 years old; 39/55/46% and 28/14/29% patients had a modified frailty score of intermediate and frail, respectively; 71/88/80% had a symptomatic relapse with at least 1 CRAB symptom; 20/28/13% patients had high cytogenetic risk; 69/72/90% patients were treated in 2-3 LOT, and 63/70/32% patients had prior exposure to a PI+IMID (P<0.05 for all). The median follow-up was 14.4 months.
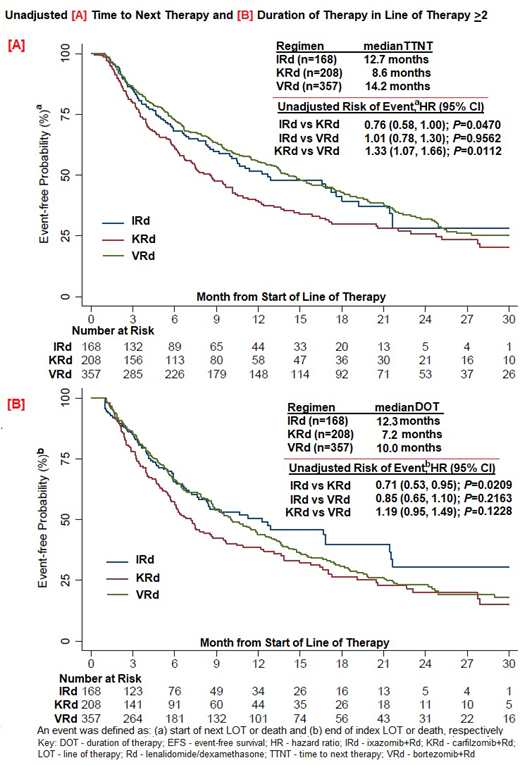
Unadjusted median DOT of index LOT was 12.3/7.2/10.0 months, with a lower risk of discontinuation of the oral PI component for I in comparison to both K and V (HR[I vs K] of 0.66 and HR[I vs V] of 0.68) and R component in the IRd vs KRd and VRd groups (HR[I vs K] of 0.64 and HR[I vs V] of 0.75) (P<0.05 for all). Unadjusted median TTNT was 12.7/8.6/14.2 months for I-/K-/V-Rd in LOT ≥2; with HR for TTNT of 0.76 (IRd vs KRd; P<0.05); 1.01 (IRd vs VRd; P=0.96); 1.33 (KRd vs VRd; P<0.05).
The adjusted HRs for TTNT I-/K-/V-Rd in LOT ≥2 were 0.82 (IRd vs KRd); 0.93 (IRd vs VRd); 1.13 (KRd vs VRd; P>0.05 for all), and were comparable between all 3 groups. A significant TTNT benefit for IRd vs KRd was observed among patients with high cytogenetic risk (HR: 0.47) and with intermediate frailtymodified score (HR: 0.60; P<0.05 for all).
CONCLUSIONS: In this real-world representative RRMM cohort, in unadjusted analyses, the IRd group had a longer DOT of the PI and R components and a longer TTNT in comparison with KRd. After adjusting for baseline confounders to separate the treatment effect, the I/K/V-Rd triplet combinations were comparable in TTNT, but IRd was significantly better than KRd in patients with high-risk cytogenetics and with an intermediate frailty score. Residual confounding is always a potential limitation of any non-randomized study. Conversely, the results of randomized, clinical trials are limited in generalizability as 40-60% of patients are excluded due to inability to meet eligibility criteria. This is especially pertinent in an elderly RRMM population as demonstrated in the real-world effectiveness results in this study.
Ref: 1. Palumbo, et al. Blood. 2015;125:2068-2074
Chari:Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Oncoceutics: Research Funding; Novartis Pharmaceuticals: Research Funding; GlaxoSmithKline: Research Funding; Array Biopharma: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Romanus:Takeda: Employment. Raju:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Other: Xcenda received funding from Takeda to complete this analyses., Research Funding; Xcenda, LLC: Employment. Cain:Takeda: Employment. Blazer:Xcenda: Employment; Takeda: Other: Xcenda received funding from Takeda for completion of this analysis, Research Funding. Farrelly:Xcenda: Employment; Takeda: Other: Xcenda received funding from Takeda for completion of this analysis, Research Funding. Stull:Takeda: Employment. Ailawadhi:Pharmacyclics: Research Funding; Cellectar: Research Funding; Celgene: Consultancy; Takeda: Consultancy; Janssen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal