Introduction:
AL amyloidosis involves deposition of abnormally folded light chains into a wide range of tissues causing end-organ dysfunction, including in the heart and kidney. Daratumumab, a CD38-targeted antibody, has recently demonstrated efficacy in producing hematologic responses in previously relapsed/refractory disease. However, data on long-term outcomes to daratumumab, including organ responses, are lacking. Here we present the largest retrospective study to date on patients with previously treated AL amyloidosis treated with daratumumab.
Methods:
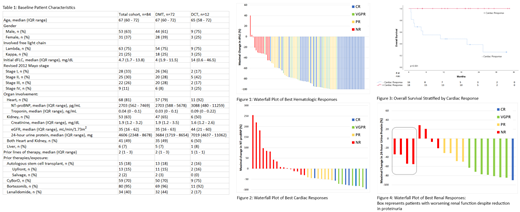
We conducted a retrospective analysis of relapsed/refractory AL amyloidosis patients treated at Stanford University from January 2016 to January 2019. Patients treated with daratumumab, either as monotherapy with dexamethasone (DMT) or in combination with other plasma-cell directed therapies (DCT) were included. Hematologic and organ responses were assessed by consensus guidelines. Hematologic responses were based on the maximal change in the difference between involved and uninvolved free light chains (dFLC). For cardiac response, a >30% and >300 pg/mL decrease in NT-proBNP for patients with initial baseline NT-proBNP ≥650 pg/mL was considered a response. A graded cardiac response metric was also explored with partial response (PR) representing 30-59% reduction, very good partial response (VGPR) ≥60% reduction, and complete response (CR) NT-proBNP <450 pg/mL as previously reported. For renal response, a >30% decrease (by at least 0.5 g/day) in 24-hour urine protein without worsening in creatinine or creatinine clearance by more than 25% in patients with at least 0.5 g/day pretreatment was considered a response. A graded renal response metric was also explored with PR representing 30-59% reduction in proteinuria, VGPR ≥60%, and CR ≤ 200 mg per 24-hour period. Survival data was analyzed using the Kaplan-Meier method. All time-to-event outcomes, including survival and organ responses, were determined from initiation of daratumumab.
Results:
Eighty-four patients were identified with baseline characteristics at start of daratumumab shown in Table 1. Median duration of follow-up was 16 months. Two-year overall survival (OS) was 83% and median OS was not reached. Median time-to-next-treatment or death was 31 months. Sixty-seven out of 80 evaluable patients (84%) achieved a hematologic response, with 47 patients (59%) achieving a VGPR or better (Figure 1). Sixty-eight patients (81%) had cardiac involvement, and among the 34 evaluable patients, 18 (53%) of evaluable patients achieved a cardiac response with a median response time of 2 months among responders. In terms of a graded cardiac response, 6 patients (18%) were able to achieve cardiac CR, 5 patients (15%) cardiac VGPR, and 7 patients (21%) PR (Figure 2). The median NT-proBNP percent reduction was 64.5% (IQR: 48.3 - 81.1%) and the median absolute reduction was 2395 pg/mL (IQR 1279.5 - 4089.5 pg/mL). Cardiac responses were associated with an improvement in OS (p<0.001, Figure 3), with landmark analysis for cardiac responses at 6-month trending towards statistical significance (100% vs. 51% at 30 months, p=0.052). Fifty-three patients (63%) had renal involvement, and among the 26 evaluable patients, 12 patients (46%) achieved a renal response with a median initial response time of 6 months among responders. Using graded response, 1 patient (4%) achieved renal CR, 7 patients (27%) renal VGPR, 4 patients (15%) renal PR, and 14 patients had no response, worsening creatinine, or were subsequently started on hemodialysis (54%) (Figure 4). The median percent reduction in proteinuria was 74.1% (IQR: 49.2 - 83.1%) and the median absolute reduction in proteinuria was 3.1 g/24 hours (IQR 2.1 - 4.9 g) among responders. There were no significant differences in OS between renal responders and non-responders.
Conclusion:
Daratumumab is highly effective in the treatment of previously treated AL amyloidosis, and a significant proportion of patients can achieve durable hematologic responses as well as improvements in organ function.
Kaufman:Janssen: Other: travel/lodging, Research Funding. Liedtke:Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; IQVIA/Jazz: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Roche: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celator: Research Funding; Caelum: Membership on an entity's Board of Directors or advisory committees; BlueBirdBio: Research Funding; Amgen/Onyx: Consultancy, Honoraria, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Agios: Research Funding.
Daratumumab in AL amyloidosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal