Introduction. The development of novel agents has improved the outcomes of multiple myeloma (MM) patients. Especially, daratumumab, an anti-CD38 monoclonal antibody which exerts therapeutic effect against MM cells through direct cell damage, antibody dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), has shown its high efficacy in clinical practice. CD38 is a transmembrane glycoprotein highly expressed in plasma cells. CD38 is also a major nicotinamide adenine dinucleotide (NAD) glycohydrase in mammalian tissues, which regulate cellular levels of NAD. However, the role of CD38 as a NAD glycohydrase (NADase) in survival of MM cells is not well understood. In the present study, we conducted CD38 enzyme activity inhibition on MM cells using a small molecule compound 78c, a specific inhibitor for NADase enzymatic activity of CD38, in order to study the role of CD38 NADase activity in MM cell survival and to examine whether CD38 enzyme inhibition could be a new therapeutic strategy of MM.
Materials and methods. MM cell lines (NCI-H929, KMS-12BM, KMS-12PE, U266) were treated with CD38 NADase inhibitor, 78c, in vitro. Viability of MM cell lines and patient-derived MM cells were analyzed by flow cytometry after 7AAD staining. MM cell lines possessing CD38 positive and negative fraction were sorted according to the CD38 expression using CD38 Micro-Beads. CD38 low MM cell lines were treated with All-trans retinoic acid(ATRA)to increase surface CD38 expression. Intracellular NAD and NADH concentrations in MM cells were analyzed using NAD / NADH assay kit. Detection of apoptosis in MM cell lines were examined by Annexin V and PI staining followed by flow cytometry analysis. Caspase inhibitor, Z-VAD-FMK, was used in combination with 78c to study the mechanism of 78c induced MM cell death.
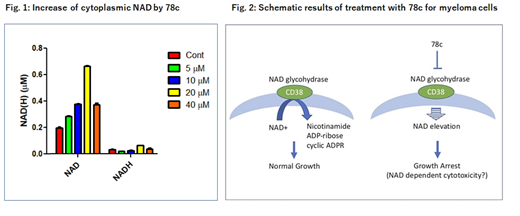
Results. 78c induced cell death in MM cell lines at low concentrations (IC50 10-20 μM). Addition of 78c to patient derived bone marrow cells showed cytotoxicity to MM cells, while toxicity to non-MM cells were limited. CD38 positive fraction of MM cell lines had better sensitivity to 78c compared to CD38 negative fraction. CD38 induction by ATRA in CD38 low MM cell lines showed increased sensitivity to 78c. These results proved that 78c efficacy correlates with surface CD38 expression. Comparison of intracellular NAD and NADH concentrations between CD38 positive and negative fractions of MM cell lines demonstrated a significant increase of NAD in the CD38 negative fraction compared to their positive counterparts, indicating that CD38 is indeed controlling the intracellular NAD concentration. Marked increase of NAD / NADH ratio was observed in 78c treated MM cell lines compared to control, proving that CD38 NADase inhibition affects intracellular NAD concentration in MM cells (Fig. 1). 78c treatment of MM cell lines significantly reduced the number of viable cells in the Annexin- / PI- region, however, addition of Z-VAD-FMK did not lead to recovery of viable cell numbers, indicating non-apototic cell death induction by CD38 NADase inhibition.
Conclusions. CD38 is the major NADase in mammalian tissues, and involved in catabolism of NAD. CD38 NADase inhibitor, 78c, inhibited the growth of MM cells at low concentrations. 78c induced cell death was found to be highly specific to MM cells and its cytotoxic effect was associated with surface CD38 expression of MM cells. Increased amount of NAD in MM cells by 78c treatment suggests that NAD elevation is associated with MM cell death induced by CD38 NADase inhibition. Since, daratumumab has limited effect against CD38 NADase activity, modulation of intracellular NAD levels by CD38 NADase inhibition could provide a novel therapeutic strategy for MM (Fig. 2).
Matsuoka:Kyowa Kirin Co., Ltd.: Research Funding; Bristol-Myers Squibb Corp.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal