Somatic mutations of CEBPA in AML are present at similar rates in all age groups and have been shown to be associated with favorable outcome. The most common cooperating variant in CEBPA mutations is a second CEBPA mutation, where the cooperation of TAD and bZip mutations lead to a highly penetrant variant which is sufficient for malignant transformation. Data suggested that only double CEBPA mutations (CEBPA-dm) were associated with favorable outcome. Currently, WHO classification of AML includes CEBPA-dm as a diagnostic entity in AML. We previously reported that in children and young adults, somatic single CEBPA mutations, which almost exclusively occurred in the bZip domain, had similar outcomes to CEBPA-dm patients. We have also shown that CSF3R and GATA2 mutations occur in childhood AML and are highly associated with CEBPA mutations. In this large analysis, we sought to define the prognostic impact of CEBPA-dm vs single bZip mutations and evaluate the impact of common co-occurring mutations.
This study included a total of 2,958 patients (age 0-29.94 years) treated on the 4 large Children's Oncology Group trials CCG2961 (n=562), AAML03P1 (n=266), AAML0531 (n=917), and AAML1031(n=1233). DNA binding domain of CEBPA (bZip domain) was analyzed by fragment length analysis and 160 patients with mutations were identified and verified with sanger sequencing (n=23 2961; n=15 03P1; n=51 0531; n=71 1031). In all cases where bZip mutations were detected, N terminal region was sequenced for identification of TAD mutations. Of the 160 cases with bZip mutation, 132 patients had a secondary TAD mutation (CEBPA-dm; 82.5%) and in the remaining 28 cases (17.5%) no TAD mutation was detected (CEBPA-sm). Comprehensive NGS data was available in a cohort of patients (n=106) and demonstrated dm vs. sm prevalence of 81% and 19% respectively, similar to the cohort overall.
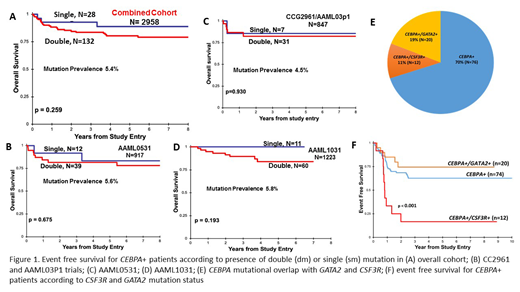
We compared clinical and biologic characteristics among CEBPA-dm vs. CEBPA-sm patients. The two groups showed no significant differences across karyotype, complete remission rates, FLT3/ITD or NPM1 status, cyto-molecular risk group, age, or diagnostic WBC. CEBPA mutations were more prevalent in Asian patients (10.5%) compared to other races (p=0.019). Evaluation of outcomes for those with CEBPA-dm vs. sm in the entire cohort demonstrated that those with CEBPA bZip mutations regardless of dm vs. sm status had identical event free survival (EFS) of 64% (Fig 1A; p=0.777). Similarly, the overall survival (OS) for CEBPA-sm was 89% vs. 81% for CEBPA-dm (p=0.259; Figure 1A). Evaluation by study cohort demonstrated that presence of bZip mutations whether as sm or dm had similar favorable clinical impact (Figure 1B-D; p=NS in each study for EFS and OS).
Analysis of CEBPA+ patients with comprehensive NGS data identified somatic mutations in GATA2 (n=20; 19%) and CSF3R (n=12; 11%)(Figure 1E). Co-occurrence of a GATA2 mutation did not impact the favorable outcome conferred by CEBPA+ on EFS or OS (p=0.356 and p=0.749 respectively; Figure 1F). However, presence of CSF3R mutation significantly modulated the clinical implications of CEBPA mutations. We previously showed an overlap with CEBPA and CSF3R mutations, which occur as SNVs in the extracellular domain or truncating mutations in the cytoplasmic domain. CSF3R mutations result in aberrant CSF3R activation and expression and have been shown to be amenable to targeted therapy with either JAK or SRC family inhibitors. Given the large number of CEBPA+ patients available for analysis, we evaluated the outcomes of patients with CEBPA+/CSF3R+ (n=12) and without CSF3R mutations (n=94). Dual mutant CEBPA+/CSF3R+ patients had a significantly inferior EFS of 17% vs. 63% (p<0.001; Figure 1F) due to high rate of relapse. Importantly, despite high relapse rates these dual mutant patients were salvageable after relapse with an OS of 73% vs. 83% for CEBPA+ patients without CSF3R (p=0.563).
In a large study of CEBPA mutations in pediatric AML, we show that patients with a bZip mutation, regardless of sm vs. dm status have a favorable prognosis. Further, we confirm the significant overlap of CEBPA and CSF3R mutations previously reported, and demonstrate that CEBPA+/CSF3R+ patients are at high risk for relapse and thus should not be considered a low risk cohort. Given the poor outcomes with current regimens, patients with CEBPA+/CSF3R+ mutations could be considered for addition of tyrosine kinase inhibitors with upfront therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal