Background:
Activating mutations of NRAS and KRAS genes are common in newly diagnosed acute myeloid leukemia (AML), occurring in 11-16% and 4-5% of patients, respectively. RAS mutations are frequently acquired at time of progression from MDS to AML and are associated with poor survival. Next generation sequencing (NGS) at diagnosis and during complete remission has shown that RAS mutations have high clearance rates with induction chemotherapy. In the CALGB 8525 study, RAS-mutant younger patients (age <60 years) randomized to treatment with high-dose cytarabine consolidation had a lower 10-year cumulative incidence of relapse when compared to RAS WT patients. We performed a single center retrospective study to determine the outcomes of NRAS and KRAS mutated AML in patients receiving induction chemotherapy.
Methods:
We retrospectively reviewed the charts of patients with newly diagnosed AML treated at Memorial Sloan Kettering Cancer Center between January 1, 2014 to May 15, 2019. Patients with pathologic confirmation of AML and treatment with induction chemotherapy were included. Age < 18 years old, treatment with a pediatric induction regimen, a diagnosis of biphenotypic AML, unknown RAS mutation status at diagnosis, or treatment an outside institution were criteria for exclusion. All patients underwent NGS from a diagnostic bone marrow aspirate (BMA) with MiSeq or MSK-IMPACT platforms. Mutations present with a variant allele frequency (VAF) ≥ 1% were retained. Response was evaluated per ELN 2017 criteria. Immunophenotypic MRD was identified in BMA by multiparameter flow cytometry. Any level of residual disease was considered MRD+. Baseline characteristics were evaluated by Fisher's exact test and Wilcoxon rank sum tests. Kaplan-Meier estimates were used to summarize OS and EFS. Multivariable cox regression, including time-dependent variables was performed on univariate factors with p<0.05.
Results:
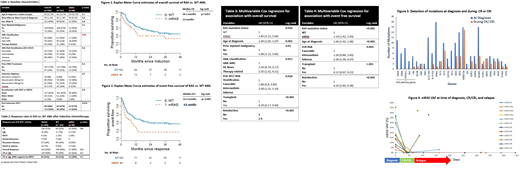
202 patients, including 162 WT and 40 RAS mutant met inclusion criteria for further analysis. Mutations in NRAS and KRAS occurred in 14%, and 8% of patients, respectively with 6 patients having co-occurring NRAS and KRAS mutations. At baseline, the RAS mutant AML cohort had a significantly greater proportion of patients with AML-MRC and a trend toward fewer patients receiving allogeneic stem cell transplant. (Table 1.) Cytogenetic abnormalities were similar among RAS and WT patients. Sequencing at diagnosis revealed an increased frequency of FLT3 TKD, RUNX1, TET2, WT1, and ETV6 mutations and a decreased frequency of FLT3-ITD and TP53 mutations in the RAS mutant cohort. Response rates and MRD negative remission rates to induction chemotherapy were similar between RAS and WT AML patients (Table 2). With a median follow up of 25 months among survivors, RAS mutant AML was associated with a significant decrease in median EFS (4.9 vs. 11.4 months, p< 0.01) and a near significant decrease in median OS (12 vs. 30.1 months, p=0.057) (Figure 1 and 2). After controlling for variables with p<0.05 on univariate analysis including age, prior myeloid malignancy, AML classification, ELN risk, transplantation, and re-induction, RAS mutation was independently associated with an increased risk of death (HR 1.85, p=0.016) and decreased EFS (HR 2.19, p< 0.01) on multivariate analyses (Tables 3 and 4). Among 77 patients with paired sequencing at diagnosis and at time of CR or CRi, all RAS mutations (n=17) were cleared (Figure 3). Additionally, other RAS pathway mutations had high clearance rates including PTPN11 (n=8, 100%), NF1 (n=3, 100%, and CBL (n= 4, 80%) (Figure 3). RAS mutation clearance also occurred in 3 out of 8 patients (38%) not achieving CR or CRi after induction. RAS mutation clearance persisted in 6 out of 10 responding patients at time of relapse.
Conclusions:
In summary, the presence of RAS mutations in patients with AML receiving induction chemotherapy was associated with decreased overall and event free survival. RAS mutant AML was enriched among patients with AML-MRC and prior myeloid neoplasms, which was also associated with decreased survival. Lastly, treatment with chemotherapy led to a high rate of RAS mutation clearance in responders that persisted at the time of disease relapse. The poor prognosis of RAS mutant AML despite RAS mutation clearance suggests that other therapies are needed in combination with chemotherapy to improve outcomes in this high-risk population.
Cai:Imago Biosciences, Inc.: Consultancy. Viny:Hematology News: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Other: Sponsored travel. Goldberg:American Society of Clinical Oncology: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Research Funding; American Society of Hematology: Research Funding; DAVA Oncology: Honoraria; Pfizer: Research Funding; Arog Pharmaceuticals: Research Funding; Abbvie: Consultancy; Daiichi-Sankyo: Consultancy, Research Funding; Celgene: Consultancy. Tallman:BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Consultancy; Cellerant: Research Funding; ADC Therapeutics: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees. Stein:Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal