
Introduction: Venetoclax (VEN) is a potent and selective inhibitor of BCL-2. It has demonstrated activity in adults with acute myeloid leukemia (AML) in combination with low-dose cytarabine (<100mg/m2/day) and hypomethylating agents. Here, we report safety and activity of VEN in combination with intermediate- and high-dose cytarabine with or without idarubicin in children and young adults with relapsed or refractory AML.
Methods: VEN was given daily for 28 days and chemotherapy was started on day 8, or earlier in cases of disease progression. Dosages of VEN and chemotherapy were escalated separately using a rolling-six design. Response to the VEN window was determined using total peripheral blood blast count or, in a subset of patients, bone marrow blast percentage as determined by flow-cytometry based minimal residual disease (MRD). Pharmacokinetics of VEN both as a single agent and in combination with chemotherapy were measured in a subset of patients treated at the maximum tolerated combination dose of VEN. Response to therapy was determined using bone marrow evaluation between days 29-50 of therapy. All patients received antimicrobial prophylaxis, typically with levofloxacin and micafungin. Azoles were prohibited during VEN administration.
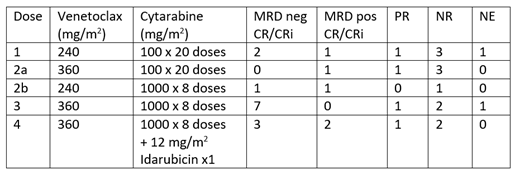
Results: Thirty-six patients aged 2-22 years were enrolled. All dose levels were tolerated. The recommended phase 2 dose of VEN in combination with high-dose cytarabine with or without idarubicin was 360 mg/m2 daily (max 600mg). One patient (treated with 240mg/m2 of VEN and intermediate-dose cytarabine) experienced a dose-limiting toxicity due to delayed count recovery and one patient died of recurrent colitis (at dose level 3) which first occurred during prior therapy and was deemed unrelated to VEN. Patients experienced a mean of 2.4 non-hematologic grade 3+ toxicities, with infections including culture-negative febrile neutropenia, sepsis, and colitis the most common toxicities. Patient-reported quality of life was similar at study entry and at the completion of cycle 1 and was within normal limits in most patients.
Among 22 patients receiving VEN with high-dose cytarabine ± idarubicin, 14 (64%) achieved a complete response (CR) or complete response with incomplete count recovery (CRi). Response to the VEN window was associated with end of cycle 1 response; 13/15 (87%) patients with a greater than 80% reduction in peripheral blasts achieved a partial response (PR; 3) or CR/CRi (10). In contrast, only 8/15 (53%) patients with less than an 80% reduction in blasts responded to combination therapy (7 CR, 1 PR).
Window response to VEN was associated with BH3 dependence as determined by cytochrome c release from leukemia cells in a flow-cytometry based assay. 5 of the 6 (83%) patients with primary BCL-2 dependence had a >80% reduction in blasts; the single patient with a poor response had a change to BCL-XL dependence at the end of cycle 1. In contrast, 4 of the 6 (66%) patients with primary BCL-XL dependence had a <80% reduction in blasts; the 2 patients with a >90% reduction had secondary BCL-2 dependence. None of the 4 patients with FLT3-ITD or point mutations responded as determined by end of cycle 1 marrow.
VEN levels were consistent across weights and ages and similar to levels seen in adults. The levels were similar between patients who did and did not receive idarubicin (mean AUC24 38.3 ± 32.7 vs. 47.3 ± 22.9 μg•h/mL).
Conclusion: VEN combined with high-dose cytarabine or high-dose cytarabine and idarubicin was well tolerated and effective in children and young adults with relapsed or refractory AML. Enrollment continues to refine estimates of response rate. VEN window response is associated with BH3 dependence and end of cycle 1 response rates. Targeting BCL-XL or FLT3 may improve response to combination therapy.
Karol:Abbvie: Other: Unrelated to this study, St. Jude has received a charitable contribution from AbbVie, Inc. The charitable contribution is not being used for clinical or research activities, including any activities related to this study.. Alexander:AbbVie: Other: travel funding. Salem:AbbVie: Employment, Other: Stock/stock options. Palenski:Abbvie: Employment, Other: Stock/ stock options. Opferman:AbbVie: Research Funding. Rubnitz:AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal