Introduction
Patients with myelodysplastic syndromes (MDS) are at risk of early death from cardiovascular complications due to the link between clonal hematopoiesis and endothelial dysfunction. EASIX (Endothelial Activation and Stress Index) has been established to predict endothelial complications after allogeneic stem cell transplantation (alloSCT). EASIX is an endothelial dysfunction related biomarker that can be easily obtained with routine laboratory markers (creatinine× LDH/thrombocytes). We analyzed whether EASIX can predict mortality in higher-risk and in lower-risk MDS patients. Since alloSCT can alter the natural history of MDS and may be an independent contributor to endothelial complications, this analysis was restricted to patients who did not undergo alloSCT during the course of their disease.
Patients and Methods
We investigated the impact of EASIX measured at first diagnosis on survival of patients with lower- and higher-risk MDS (no allogeneic transplantation) in two independent institutions: n=192 (training cohort) and n=333 (validation cohort). For the purposes of this study, patients with low and intermediate-1 according to IPSS and very low and low-risk according to IPSS-R, respectively, were considered to have lower-risk MDS, while patients with intermediate-2 and high-risk according to IPSS and intermediate, high and very-high according to IPSS-R, respectively, were considered to have higher-risk MDS. Serum markers of endothelial cell distress were measured and correlated to EASIX.
Results
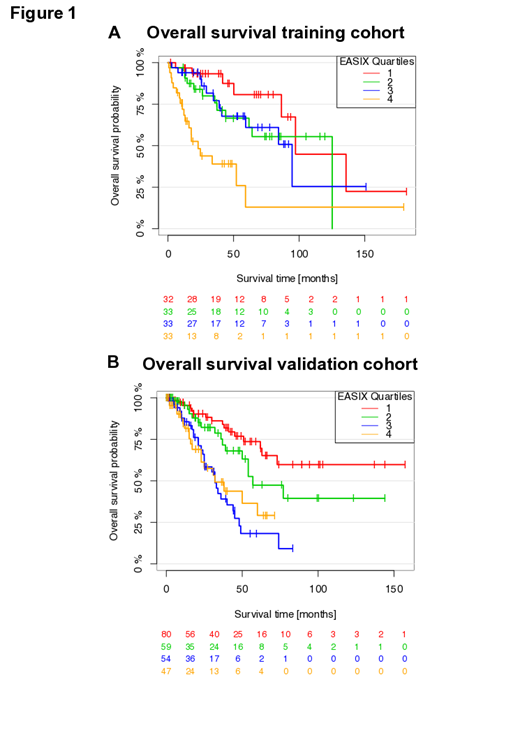
While no effects of EASIX on survival were observed in higher-risk patients, EASIX was associated with shorter survival in patients with lower-risk MDS in both cohorts (univariate: training cohort: hazard ratio (HR): 1.46; 95% confidence interval (CI) 1.24-1.71; p-value < 0.001 / validation cohort: HR 1.31 [1.17-1.48]; p-value < 0.001). We visualized this continuous effect of EASIX in lower-risk disease by grouping patients into quartiles according to EASIX (Figure 1 A and B). In both cohorts, patients in the highest quartile had a shorter survival compared to patients in lower quartiles. Confining our analysis to lower-risk patients who did not develop AML within the observation period, EASIX similarly predicted OS in both cohorts (training: n=78, no. of events n=17, HR per log2 increase 1.43 [1.05-1.94]; p=0.02; validation: n=267, no. of events n=93, HR per log2 increase 1.33 [1.21-1.47]; p<0.00). Multivariate Cox regression analysis and prediction error analyses confirmed that EASIX remained a significant predictor of survival after adjustment for age, sex, cytogenetic abnormalities and bone marrow blasts in lower-risk patients. The model of the training cohort could be validated. No effect of EASIX on survival was found in higher-risk patients from both cohorts in univariate and multivariate analysis. Serum levels of Angiopioetin-2 correlated significantly with EASIX whereas S100A9 or IL-1b did not. However, we observed a strong intra-pathway correlation of S100A9 with IL1b, IL18, IL37, and HMGB1.
Conclusion
We introduce EASIX as an easily accessible and independent predictor for survival in patients with lower-risk MDS. The strong correlation of EASIX and ANG2 underlines the endothelial nature of this biomarker.
Germing:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria. Kobbe:Neovii: Honoraria, Other: Travel support; Pfizer: Honoraria, Other: Travel support; Medac: Honoraria, Other: Travel support; Abbvie: Honoraria, Other: Travel support; Biotest: Honoraria, Other: Travel support; Jazz: Honoraria, Other: Travel support; Celgene: Honoraria, Other: Travel support, Research Funding; Roche: Honoraria, Other: Travel support; Amgen: Honoraria, Other: Travel support, Research Funding; MSD: Honoraria, Other: Travel support; Novartis: Honoraria, Other: Travel support; Takeda: Honoraria, Other: Travel support. Kaivers:Jazz Pharmaceuticals: Other: Travel Support. Merz:Janssen: Other: Travel grants; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Abbvie: Other: Travel grants; Celgene: Other: Travel grants; Takeda Vertrieb GmbH: Other: Travel grants, Research Funding. Dreger:MSD: Membership on an entity's Board of Directors or advisory committees, Other: Sponsoring of Symposia; Neovii, Riemser: Research Funding; AbbVie, Gilead, Novartis, Riemser, Roche: Speakers Bureau; AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal