Introduction:
Peripheral T cell lymphoma (PTCL) is a clinically and molecularly heterogenous disease that comprises 15% of all Non-Hodgkin's lymphoma. PTCL patients' have poor durability of response to existing therapies and inferior overall survival (OS), indicating an unmet need to identify new targeted therapies. Elotuzumab, is a humanized IgG1 monoclonal antibody targeting the transmembrane receptor signaling lymphocytic activation molecule family 7 (SLAMF7) expressed on lymphocytes. Notably, SLAMF7 (CS1/CD319) is expressed on 92% of nasal-Natural Killer/T-cell lymphoma and 44% of angioimmunoblastic T lymphoma (AITL)-subtypes of PTCL (Hsi ED et al 2008 Blood 112:1779; Elmaagacli AH et al 2019 Leukemia & lymphoma). However, SLAMF7 is often down-modulated and expressed at low levels on malignant T cells. Expression of SLAMF7 on NK and plasma B cells is transcriptionally upregulated by PRDM1/BLIMP1 (Kim JR et al 2016 Immunobiology 221: 31-39), which in turn is frequently hypermethylated in T-cell lymphoma (Zhang et al 2017 Hematological Oncology 35:645-654). We hypothesized that treatment of malignant T-lymphoma cells with epigenetic agents that are expected to remove methylation and upregulate gene expression would improve sensitivity to elotuzumab.
Methods and Results:
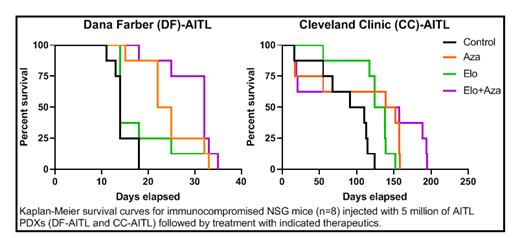
Our first objective was to determine if 5-azacytidine treatment of T-lymphoma cells resulted in demethylation-induced PRDM1 expression and a subsequent increase in SLAMF7 expression. We performed flow cytometry analysis to assess SLAMF7 expression on CD45+CD3+ AITL T-lymphoma cells engrafted in NSG mice, treated or not with 5-azacytidine (2 mg/Kg). The AITL T-lymphoma cells from the 5-azacytidine-treated group showed increased SLAMF7 expression compared to the untreated group. Gene expression analysis by real-time polymerase chain reaction (RT-PCR) indicated increased transcription of PRDM1 and SLAMF7 in the 5-azacytidine-treated group compared to the untreated control. Next, we tested the therapeutic efficacy of elotuzumab (5 mg/Kg) and 5-azacytidine as single agents and in combination using two separate patient-derived xenografts (PDXs) of AITL-subtype. We observed markedly extended survival with the elotuzumab-azacytidine combination therapy when compared to elotuzumab alone. In comparison to the control groups, the 5-azacytidine-elotuzumab combination extended survival from 18 to 35 days and from 124 to 195 days for mice engrafted with the Dana Farber-AITL (DF-78024) and Cleveland Clinic-AITL PDX, respectively. Together these data suggest that demethylation of the PRDM1 gene allows for increased expression of SLAMF7, thereby sensitizing the T-cell lymphoma to elotuzumab.
Notably, T-lymphoma cells of cutaneous T-cell lymphoma (CTCL) and PTCL, not otherwise specified (PTCL, NOS) similarly upregulated PRDM1 gene expression after 5-azacytidine treatment. 5-azacytidine treatment also increased SLAMF7 gene and protein expression assessed by RT-PCR and immunoblotting. Our data indicated that it may be possible to upregulate the elotuzumab target SLAMF7 in most clinical subtypes of T-cell lymphomas, by altering the methylation of PRDM1. We are currently investigating the functional consequences of 5-azacytidine treatment on the efficacy of elotuzumab using PDX models of distinct T-lymphoma subtypes.
Conclusion
This study identifies 5-azacytidine-induced molecular alterations in T-cell lymphoma that improve sensitivity to elotuzumab. Improved understanding of the underlying molecular mechanism, together with information of its broader applicability across T-lymphoma molecular subtypes will have significant clinical impact on the targeted therapies considered for PTCL.
Hsi:Jazz: Consultancy; Eli Lilly: Research Funding; Cleveland Clinic&Abbvie Biotherapeutics Inc: Patents & Royalties: US8,603,477 B2; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal