Introduction
The outcome HSCT in a cohort of children with chemorefractory acute leukemia (AL) is poor. The incidence of relapse exceeds 50% and survival varies from 10 to 40%. Additional attempts at remission induction with various combinations of chemotherapy are unlikely to improve the outcome and may contribute to toxicity. We hypothesized that personalized targeted therapy combined with high-dose preparative regimen may improve the outcome of alloHSCT in a cohort of pediatric patients with refractory AL. Bcl-2 and CD38 were chosen as potential targets due to frequent expression in pediatric acute leukemia, availability of marketed targeted therapies, venetoclax and daratumumab, and expected non-overlapping toxicity profile of these agents and the conditioning regimen.
Materials and methods
A total of 26 pts with chemorefractory acute leukemia (AML-18, T-ALL-6, ABL-2, primary refractory - 9, refractory relapse - 17, 18 male, 8 female, median age 11.3 years), underwent HSCT between November 2017 and May 2019. All patients were transplanted from haploidentical donors, all patients had active disease (AD) at the moment of SCT. For 19 (73%) pts it was the first alloHSCT, for 7 (27%) pts it was the second HSCT. Median bone marrow leukemia burden before cytoreduction was 11% (3-90). Bcl-2 expression on the tumor cells was detected in 23 pts with the median expression of 58% (1.5-99), CD38 expression was detected in 24 pts with the median expression of 88% (11-100). Thirteen pts received treosulfan-based conditioning and 13 - TBI-based (12 Gy). TBI was used among patients with second HSCT (n=7), patients with T-ALL (n=4) and patients with massive extramedullary disease (n=2). GvHD prophylaxis included tocilizumab at 8 mg/kg on day -1 and abatacept at 10 mg/kg on day -1, +7, +14, +28. According to the expression of Bcl-2 and CD38 on tumor cells, 24pts (92%) received daratumumab (anti-CD38 monoclonal antibody) on day -7 at 10-16 mg/kg, 21 pts (81%) received venetoclax at 300 mg/m2/day on days -7 to -2 and 12 pts additionally received plerixafor at 240 mcg/kg x 3 on days -7 to -5. TCRαβ+/CD19+ depletion of PBSC with CliniMACS technology was implemented in all cases. The median dose of CD34+ cells in the graft was 9.5 x106/kg (range 4.9-13.3), αβ T cells - 26.5x103/kg (range 5.6- 84). Modified (CD45RA-depleted) donor lymphocyte infusions (DLI) were administered prophylactically in all pts.
Results
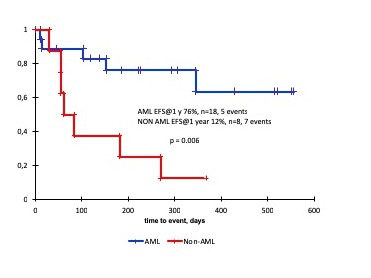
Two patients died before engraftment due to septic event. Primary engraftment was achieved in 24 of 24 evaluable pts (100%), the median time to neutrophil and platelet recovery was 12 and 13 days. All pts achieved complete remission on day +30. There were no significant toxic effects after targeted therapy, 4 pts (21%) had daratumumab-related infusion reactions, one patient with T-ALL died after engraftment due to septic event. aGVHD grade I-II developed in 5 pts (20%), two of them required systemic immunosuppressive therapy. There were no cases of aGVHD > grade II, neither cases of cGVHD. The median NK- cells count by the day +30 was 0.07 x 106/ml (range 0.01- 0.4), the median levels of αβ T cells and gd T cells were 0.018 x 106 /ml (range 0 - 1.1) and 0.015 x 106 /ml (range 0,01 - 1.3), respectively. Nine (37.5%) pts (5 with T-ALL, 1 with ABL, 3 with AML) relapsed, median interval to relapse was 103 days (55-345). At the moment of reporting 14 (53%) patients are alive in complete remission with a median follow up of 12.7 months (1.9-23m). Event-free and overall survival at 1 year for AML are 76% (SE13) and 76% (SE14), all patients with T-ALL and one with ABL died of disease progression.
Conclusion
We suggest that addition of venetoclax and daratumomab to the backbone of myeloablative haploidentical HSCT with αβ T cell depletion is not associated with increased toxicity and may lead to improved outcome in a cohort of pediatric patients with chemorefractory AML. In a subgroup of patients with T-ALL this approach does not seem to produce visible benefit. This approach can be further tested in a prospective trial with the goal to increase the anti-leukemic efficacy of HSCT.
Maschan:Miltenyi Biotec: Other: lecture fee.
venetoclax daratumomab plerixafor
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal