Background:
CTL019 is a therapy derived from autologous T cells expressing a CD19-specific chimeric antigen receptor (CAR) that was approved by the FDA in August 2017 (tisagenlecleucel). Complete and durable remissions have been seen in the setting of pediatric and young adult patients with relapsed and refractory B cell acute lymphoblastic leukemia (ALL) (Maude NEJM 2018). Initial case reports suggested that there may be differential outcomes mediated by cytogenetic characteristics of the leukemia at CAR T cell infusion. Here, we report results from a single institution experience of 112 patients.
Methods:
Patients with relapsed/refractory ALL were identified as having received CTL019 either in the context of a clinical trial (NCT02906371) or commercial product (tisagenlecleucel) at Children's Hospital of Philadelphia from October 2016 to April 2019. Patients who received prior CAR T therapy were excluded. Demographic, cytogenetic, and outcome data were manually abstracted from the medical record or clinical trial datasets. High risk lesions were defined as MLL(KMT2A) rearrangements, Philadelphia-chromosome (Ph+), Ph-like, hypodiploidy, and TCF3/HLF fusion. Favorable cytogenetics were defined as the presence of hyperdiploidy or ETV6/RUNX1fusion and intermediate were defined as iAMP21, IKZF1deletion, or TCF3/PBX1. Patients were classified according to their highest risk cytogenetic characteristic and stratified by cytogenetic risk category present at CAR T cell infusion. Relapse-free survival (RFS) and overall survival (OS) was described for cohorts with more than 10 patients.
Results:
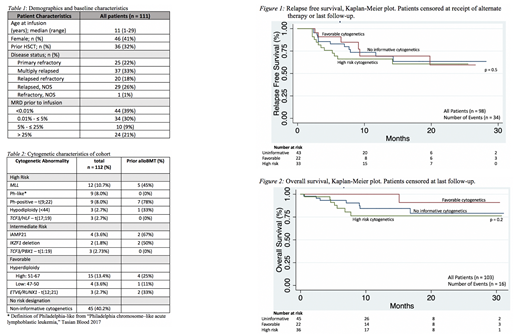
One hundred and twelve patients were included in the analysis, with a median age of 11 years (range 1-29) at infusion, of which 32% had had a previous allogeneic hematopoietic stem cell transplant (alloHSCT). Disease burden at the time of CTL019 infusion was heterogenous, with 61% having detectable disease in the bone marrow and 21% having more than 25% blasts by flow cytometry. Thirty-six patients (32%) had leukemias with high-risk genetic lesions at infusion, including 12 with MLL rearrangements and 18 with Ph+ or Ph-like lesions (Table 2). Thirty-one patients (28%) had hyperdiploidy or ETV6/RUNX1; 3 additional were in conjunction with high-risk cytogenetics (t(17;19) and 2 with Ph+), and 3 in the setting of intermediate-risk cytogenetics (iAmp21, TCF3/PBX1, IKZF1deletion). Figure 1 demonstrates RFS for those patients in remission at day 28 following infusion, stratified by cytogenetic risk category. Complete remission (CR) rate in the high-risk cytogenetics group was 94%. RFS at 12 months was 69% (0.50-0.82), 69% (0.40-0.86), and 67% (0.48-0.80) for non-informative, favorable, and high-risk cytogenetic groups, respectively. Figure 2 shows OS of patients infused with CTL019, again stratified by cytogenetic categories of interest, with a maximum follow-up time of 30 months. OS at 12 months was 84% (0.68-0.93) and 76% (0.56-0.88) for the non-informative and high-risk cytogenetic groups, respectively. There were no deaths in that time period for the favorable risk category. There was no statistically significant difference in RFS or OS for patients with high-risk cytogenetics. The intermediate-risk cytogenetics group (n<10) was excluded from these analyses.
Conclusion:
Durable remissions can be achieved with CTL019 across several high-risk cytogenetic subtypes of B-ALL. Stratifying outcomes by cytogenetic risk category in this unadjusted analysis does not show a statistically significant difference in either RFS nor OS. Further investigation is needed to parse out the contribution of individual cytogenetic lesions as well as the effects of other relapse and survival risk factors at play.
Rheingold:Novartis: Consultancy; Pfizer: Research Funding. Callahan:Novartis: Consultancy. Hunger:Bristol Myers Squibb: Consultancy; Amgen: Consultancy, Equity Ownership; Jazz: Honoraria; Novartis: Consultancy. Grupp:Novartis: Consultancy, Research Funding; Roche: Consultancy; GSK: Consultancy; Cure Genetics: Consultancy; Humanigen: Consultancy; CBMG: Consultancy; Novartis: Research Funding; Kite: Research Funding; Servier: Research Funding; Jazz: Other: study steering committees or scientific advisory boards; Adaptimmune: Other: study steering committees or scientific advisory boards. Maude:Kite: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal