Introduction
Persistence of leukemia stem cells (LSCs) in patients with Acute Myeloid Leukemia (AML) achieving complete remission (CR) after chemotherapy leads to disease recurrence and poor outcome. Therefore, the identification of LSCs driving resistance to therapy represents an important challenge. LSCs reside within the CD34+/CD38- cells and it has been recently demonstrated that co-expression of CD99 allows for separation of LSCs from functionally normal hematopoietic stem cells in AML. We showed that the presence of a CD123/CD99/CD25+ population within CD34+ cells strongly correlates with FLT3-ITD-positivity. The aim of this study was to deeply characterize the molecular profile of CD34+/CD123+/CD99+/CD25+ LSCs to shed light on the subclonal architecture of FLT3-ITD+ AML and to track the expansion of mutated clones.
Methods
Molecular status of FLT3-ITD and NPM1 were investigated at the DNA level in a cohort of 150 de novo AML patients at diagnosis and at relapse, using standard procedures. Clonal evolution of FLT3-ITD mutations was studied at diagnosis in 14 FLT3-ITD mutated AML on sorted bone marrow populations, purified by the Cytoflex High-Speed Cell Sorter using a sequential gating strategy, to separate CD34/CD123/CD99/CD25+ LSCs-, CD34+ precursors (CD123/CD99/CD25-) and T-lymphocytes. Moreover, to characterize the genetic profile of FLT3-ITD-mutated clones, DNA targeted sequencing was performed on 22 cell populations isolated from 7 AML patients, using the Oncomine™ Myeloid Research Assay panel on the Ion Torrent™ S5 sequencer.
Results
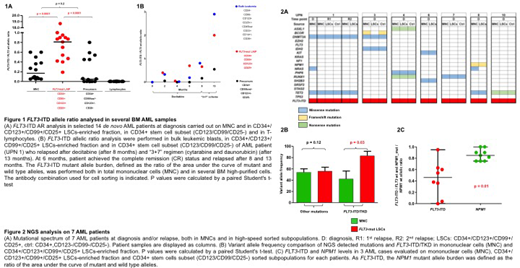
39 of 150 AML cases were FLT3-ITD+, at a median allelic ratio (AR) of 0.36 (range 0.05-7.9). The FLT3-ITD AR was significantly higher within the rare LSCs compartment as compared to MNC (median AR: 0.78, range 0.42-19.3, vs 0.09, range 0.05-0.6m p<0.0001, n=14), and to CD34+/CD123+/-/CD99low/-/CD25- precursors (Ctrl) (median AR=0.02, range 0-0.59) (p=<0.0001) (Fig 1A). In particular, a FLT3-ITD mutated minor subclone was present (median AR=0.05, range 0.05-0.08) in the MNC of 6 patients, and was highly enriched in the corresponding CD34+/CD123+/CD99+/CD25+ sorted LSCs (median AR=0.5, range 0.4-0.8). FLT3-ITD positive clones may also persist in sorted LSCs at the time of complete remission, being undetectable using standard techniques and driving later relapse (UPN1, Fig 1B). Furthermore, FLT3-ITD LOH may occur during disease progression, leading to AR >1, while the number of LSCs do not seem to expand (Fig 1B). These data confirm that LSCs may persist at rare frequency during progression, still representing the treatment-resistant FLT3-ITD reservoir, driving disease relapse with expansion of more mature, FLT3-ITD positive populations.
Aiming at shedding light on the molecular heterogeneity and subclonal structure of AMLs, using targeted NGS we compared the mutational profiles of MNCs to that of highly purified LSCs and/or to the CD34+/CD123-/CD25-/CD99- counterpart (n=7 patients). In 4 cases the same mutation pattern was present in both MNCs and LSCs. In 3 cases we found additional mutations in NRAS, BCOR and KRAS in MNCs, indicating acquisition of other transforming events during maturation (Fig 2A). Furthermore, the variant allele frequency (VAF) of mutations was similar in MNCs and LSCs (p=0.12), while FLT3-ITD and TKD burden was enriched in LSCs (p=0.03) (Fig 2B). Conversely, the NPM1 mutation burden remained almost stable in the different cell subsets (Fig 2C). In conclusion, our study shows that FLT3 mutations occur in early LSCs characterized by the CD34+/CD123+/CD99+/CD25+ immunophenotype, which represent the leukemic reservoir. These data may be the rational for CD99-targeted treatments in FLT3-ITD mutated AML to achieve durable disease eradication.
Venditti:Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy. Buccisano:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal