Background: Overexpression of the anti-apoptotic BCL-2 protein promotes multiple myeloma (MM) cell survival. Venetoclax (Ven) is a highly selective, potent, oral BCL-2 inhibitor that induces apoptosis and has shown synergistic activity with bortezomib (B) and dexamethasone (d). Phase 1 studies in relapsed/refractory (RR) MM demonstrated encouraging clinical efficacy of Ven + d in t(11;14) MM and in a broader patient (pt) population in combination with Bd. Recent results from the Phase 3 BELLINI study of Ven vs placebo (Pbo) + Bd in pts with RRMM demonstrated that pts treated with Ven + Bd had improved clinical response rates and progression-free survival (PFS) vs Pbo, although the overall survival (OS) result was in favor of Pbo. Subgroup analyses showed different efficacy and survival outcomes based on tumor cytogenetics and BCL-2 expression. Results of pre-specified subgroup analyses and additional retrospective correlative biomarker analyses in the Phase 3 BELLINI study are described herein.
Methods: BELLINI (NCT02755597) was a randomized, double-blind, multicenter Phase 3 study of Ven or Pbo + Bd in pts with RRMM who received 1-3 prior therapies and were either sensitive or naïve to PIs. Pts were randomized 2:1 to receive Ven 800 mg/day or Pbo + Bd. The following biomarker analyses were performed by central laboratory assessments of pre-treatment tumor samples: BCL-2 protein expression by immunohistochemistry (IHC) analysis of bone marrow (BM) core biopsies; BCL2 gene expression by quantitative PCR (qPCR) and cytogenetic abnormalities by interphase fluorescence in situ hybridization (FISH) analysis of CD138-enriched BM mononuclear cells. Correlation between BCL-2 (protein and gene) expression, cytogenetics, and outcomes were examined by Kruskal-Wallis tests and by hazard ratio (HR) using the Cox proportional hazard model.
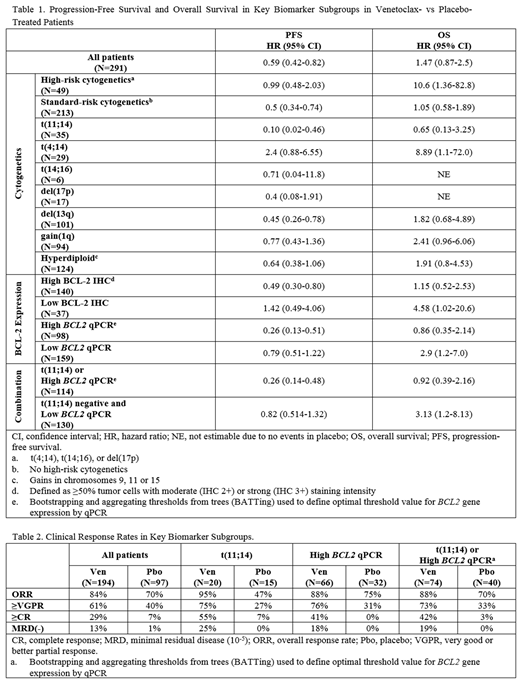
Results: As of the data cut-off of 18 Mar 2019, 291 pts were randomized, 194 to the Ven arm and 97 to the Pbo arm. Out of the 291 pts randomized, 177 pts (61%) were evaluable by IHC, 257 pts (88%) by qPCR, and 262 pts (90%) by FISH. A broad range of BCL2 gene expression was observed (median 2-DCt: 0.212 [range: 0-5.21]), which strongly correlated with protein expression (median 2-DCt 0.115 in BCL-2 IHC Low vs 0.277 in BCL-2 IHC High, p=0.0021). t(11;14) MM had the highest levels of BCL-2 expression (23/23 BCL-2 High by IHC; median 2-DCt 0.406 vs 0.212 in t(11;14) negative, p=0.0132), however high BCL-2 expression was not limited to the t(11;14) subgroup. Univariate analyses showed higher BCL2 expression in pt tumor samples with del(13q) (median 2-DCt 0.333 vs 0.159 in pts without del(13q), p=0.0008) and gain(1q) (median 2-DCt 0. 295 vs 0.180 in pts without gain(1q), p=0.0059). Bootstrapping and aggregating thresholds from trees (BATTing) was used retrospectively to identify an estimated threshold value for BCL2 expression (2-DCt ≥0.323) that could provide optimum selection of pts with maximum improvement in PFS when treated with Ven+Bd.
Biomarker subgroups with the greatest PFS improvement were t(11;14) (HR=0.10; 95% CI: 0.02-0.46, p=0.003) and High BCL2 by qPCR (HR=0.26; 95% CI: 0.13-0.51, p<0.001; Table 1). Since the t(11;14) and High BCL2 patient populations do not completely overlap (20% of High BCL2 pts were t(11;14) and 54% of t(11;14) were High BCL2), a combined subgroup analysis was performed. For pts with t(11;14) or High BCL2, the median PFS was not reached in the Ven arm vs 9.9 mo in Pbo (HR=0.26, 95% CI: 0.14-0.48, p<0.001; Table 1). Higher overall response (ORR, 88% vs 70%), very good partial response or better (≥VGPR, 73% vs 33%), and complete response or better (≥CR, 42% vs 3%) rates were observed in the Ven vs Pbo arm (Table 2). Minimal residual disease negativity (MRD-, 10-5) rate was also higher for t(11;14) or High BCL2 pts in the Ven vs Pbo arm (19% vs 0%). Median overall survival (OS) was not reached in either arm but was similar between treatment arms for the combined group with t(11;14) or High BCL2 pts (HR=0.92, 95% CI=0.39-2.16, p=0.85). In contrast, in the t(11;14) negative and Low BCL2 pts, OS favored Pbo (HR=3.13, 95% CI=1.2-8.13, p=0.019; Table 1).
Conclusions: Adding Ven to Bd demonstrates significant efficacy in pts with RRMM harboring either t(11;14) or tumor cells expressing high levels of BCL2. The benefit-risk profile appears to be favorable in these subsets of pts and additional studies to gain further safety and efficacy information are warranted.
Harrison:GSK: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cavo:celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. De La Rubia:AMGEN: Consultancy; Takeda: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Celgene Corporation: Consultancy. Popat:Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses; Janssen: Honoraria, Other: travel support to meetings; GSK: Consultancy, Honoraria; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Other: travel, accommodations, expenses. Gasparetto:Janssen: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; BMS: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; Celgene: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed . Hungria:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salwender:Amgen: Honoraria, Other: Travel or accommodations; Bristol-Myers Squibb: Honoraria, Other: Travel or accommodations; Janssen Cilag: Honoraria, Other: Travel or accommodations; Sanofi: Honoraria, Other: Travel or accommodations; Celgene: Honoraria, Other: Travel or accommodations; AbbVie: Honoraria; Takeda: Honoraria, Other: Travel or accommodations. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Spencer:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Secura Bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics Australia: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. O'Dwyer:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy; GlycoMimetics Inc: Research Funding; BMS: Research Funding; Onkimmune: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garg:Janssen: Honoraria; Novartis, Janssen: Research Funding; Janssen, Takeda, Novartis: Other: Travel expenses. Punnoose:Roche: Other: Stock/stock options; Genentech, Inc.: Employment. Jalaluddin:AbbVie: Employment, Other: Stock/stock options. Jia:AbbVie: Employment, Other: Stock/stock options. Yang:AbbVie: Employment, Other: Stock/stock options. Sun:AbbVie: Employment, Other: Stock/stock options. Ward:AbbVie: Employment, Other: Stock/stock options. Maciag:AbbVie: Employment, Other: Stock/stock options. Ross:AbbVie: Employment, Other: Stock/stock options. Kumar:Takeda: Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding.
Venetoclax is a BCL-2 inhibitor that is FDA-approved in some indications. This presentation will focus on venetoclax for treatment of multiple myeloma, which is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal