
Uproleselan (GMI-1271) is a novel antagonist of E-selectin, an adhesion molecule expressed on endothelial cells. E-selectin is expressed transiently in the normal vasculature during an inflammatory response and constitutively in the bone marrow (BM). Binding of E-selectin (E-sel) to sialyl Lex, the E-sel ligand (E-sel-L), on the leukemic cell surface activates cell survival pathways and promotes chemotherapy resistance in AML. In preclinical models, blockade of E-selectin with uproleselan disrupts the activation of cell survival pathways and enhanced the efficacy of chemotherapy across multiple AML tumor models. Furthermore, uproleselan protected against chemotherapy-induced mucositis by regulating macrophage trafficking to the site of injury in the gut lining. A previous phase I/II study of uproleselan added to chemotherapy in patients with untreated AML (older adults ≥60 yrs) and relapsed/refractory AML (≥ 18yrs) showed promising remission rates (CR/CRi) and survival outcomes, and reduced rates of mucositis. In the newly diagnosed older patients, high remission rates were achieved overall (CR/CRi 72%) and for the high risk subgroup with sAML (CR/CRi 69%). In this phase 2/3 study, we will test the addition of uproleselan to a standard daunorubicin/cytarabine regimen in older adults with previously untreated AML.
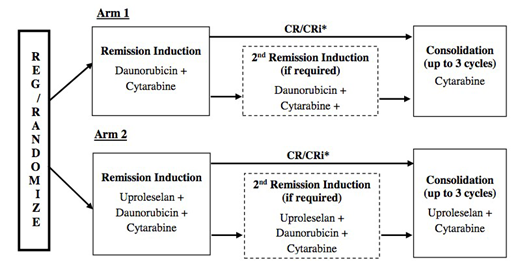
The study will enroll patients age ≥ 60 yrs with untreated acute myeloid leukemia. Patients with acute promyelocytic leukemia, activating mutations in FLT3, or evidence of central nervous system involvement are excluded. Subjects are randomized to 7+3 induction (cytarabine + daunorubicin) +/- uproleselan. Subjects achieving a CR/CRi may receive up to 3 cycles of consolidation with intermediate dose cytarabine 2 gm/m2 IV d1-5 +/- uproleselan. The primary phase II objective is to compare the event-free survival (EFS). A sample size of 262 patients was selected for the phase II to detect an improvement in median EFS from 7 months to 11 months (HR= 0.64) with > 95% power, using a log rank test. The phase III primary objective will compare overall survival (OS). For the phase III, a sample size of 335 evaluable patients per arm (670 total inclusive of patients enrolled in phase II) will provide >90% power to detect an improvement in median OS from 12 months to 16 months (HR= 0.75), using a log-rank test. Correlative studies will measure E-selectin ligand on AML blasts and soluble E-selectin and will also measure minimal residual disease after remission induction by multiparameter flow cytometry. A comprehensive geriatric assessment will identify baseline measures associated with EFS and develop a risk model to predict OS among older adults receiving intensive AML therapy. The study is endorsed by SWOG and ECOG-ACRIN and opened to enrollment on 1/16/2019.
Uy:Astellas: Consultancy; Pfizer: Consultancy; Curis: Consultancy; GlycoMimetics: Consultancy. Dinner:Agios: Consultancy; Pfizer: Consultancy; AstraZeneca: Consultancy. Strickland:Jazz: Consultancy; Sunesis Pharmaceuticals: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Astellas Pharma: Consultancy; AbbVie: Consultancy. Liesveld:Onconova: Other: Data safety monitoring board; Abbvie: Membership on an entity's Board of Directors or advisory committees. Byrd:Acerta: Research Funding; Acerta: Research Funding; Genentech: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Ohio State University: Patents & Royalties: OSU-2S; BeiGene: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Novartis: Other: Travel Expenses, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Genentech: Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Ohio State University: Patents & Royalties: OSU-2S; Ohio State University: Patents & Royalties: OSU-2S; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau. Stone:Argenx, Celgene, Takeda Oncology: Other: Data and Safety Monitoring Board/Committee: ; AbbVie, Actinium, Agios, Argenx, Arog, Astellas, AstraZeneca, Biolinerx, Celgene, Cornerstone Biopharma, Fujifilm, Jazz Pharmaceuticals, Amgen, Ono, Orsenix, Otsuka, Merck, Novartis, Pfizer, Sumitomo, Trovagene: Consultancy; Novartis, Agios, Arog: Research Funding.
Uproleselan in AML
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal