Introduction: Inotuzumab ozogamicin (InO) is a CD22-directed antibody-calicheamicin conjugate. Patients (pts) with relapsed or refractory (R/R) B cell acute lymphoblastic leukemia (ALL) treated with InO vs standard of care chemotherapy (SC) had significantly better response, and improved survival in INO-VATE (NCT01564784). Based on central laboratory assessment, this favorable benefit-risk profile of InO was independent of leukemic blast CD22 positivity (≥90% vs <90%) and CD22 receptor density (quantified as Molecules of Equivalent Soluble Fluorochrome, quartile analysis). As CD22 is usually assessed in local labs in clinical practice, this study uses INO-VATE data to investigate the relationship between baseline CD22 positivity assessed by local labs and the efficacy and safety of InO vs SC.
Methods: Adult pts (≥18 yrs) with R/R CD22-positive (based on local or central lab results) ALL in salvage 1 or 2 were randomized to InO (n=164) or SC (n=162) (details: NEJM 2016;375:740-53). InO starting dose was 1.8 mg/m2/cycle (0.8 mg/m2 on Day 1; 0.5 mg/m2 on Days 8 & 15 of a 21-28-day cycle for ≤6 cycles) and reduced to 1.5 mg/m2/cycle for pts with complete remission (CR) or CR with incomplete hematologic recovery (CRi). SC included fludarabine/cytarabine [Ara-C]/granulocyte colony-stimulating factor, Ara-C plus mitoxantrone, or high-dose Ara-C. CD22 positivity (% of leukemic blasts expressing CD22) was measured at screening by flow cytometry or immunohistochemistry. Efficacy (CR/CRi, minimal residual disease [MRD, assessed in central labs] among responders, overall survival [OS], progression-free survival [PFS], and duration of remission [DoR]) and safety outcomes were analyzed by CD22 positivity quartiles, Quartile 1 (Q1) having the lowest and Q4 the highest CD22 positivity. Data cutoff: 04Jan2017. P-values are 1-sided.
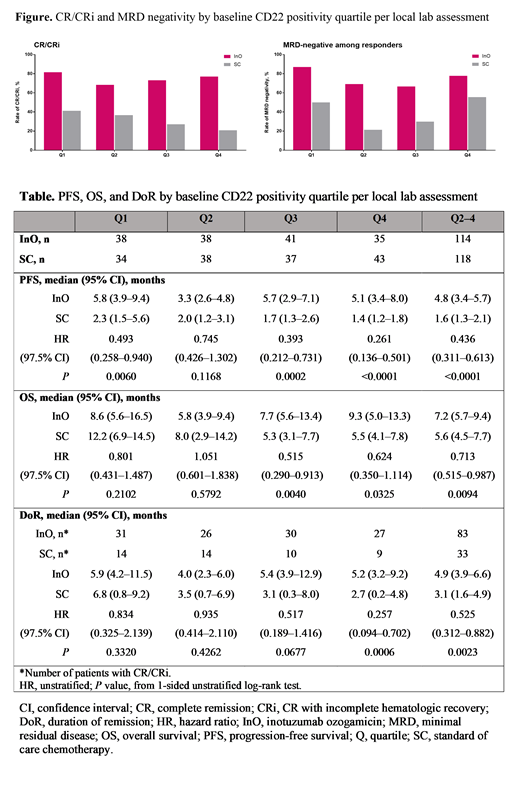
Results: Baseline CD22 positivity per local lab was available for 152 pts per arm. CD22 positivity (%) was comparable between treatment arms for each quartile; median (range) was 21.9 (1.0-39.4) for InO vs 23.9 (0.0-39.0) for SC in Q1, 55.5 (40.0-68.9) vs 56.3 (40.0-66.0) in Q2, 84.0 (70.0-92.0) vs 84.0 (70.0-92.9) in Q3, and 98.0 (93.0-100.0) for both arms in Q4. CR/CRi rates showed no difference among quartiles in the InO (P=0.5906) or SC arm (P=0.2061), and were significantly higher with InO vs SC for all quartiles (Q1: 81.6% vs 41.2%, P=0.0002; Q2: 68.4% vs 36.8%, P=0.0029; Q3: 73.2% vs 27.0%, P<0.0001; Q4: 77.1% vs 20.9%, P<0.0001) (Figure). MRD negativity rates in responders were also higher with InO vs SC and the differences in the lower quartiles (Q1-Q3) were significant (Q1: 87.1% vs 50.0%, P=0.0121; Q2: 69.2% vs 21.4%, P=0.0048; Q3: 66.7% vs 30.0%, P=0.0486; Q4: 77.8% vs 55.6%, P=0.1930) (Figure). PFS, DoR, and OS were longer with InO vs SC. The benefit of InO over SC was more evident in the higher quartiles (Q2-4); hazard ratio (97.5% Cl) was 0.44 (0.31-0.61) for PFS, 0.53 (0.31-0.88) for DoR, and 0.71 (0.52-0.99) for OS (Table).
Cytopenias were the most common ≥grade 3 adverse events in the InO arm, with similar rates across the quartiles (Q1-Q4: neutropenia: 52.6%, 47.4%, 39.0%, and 48.6%; thrombocytopenia: 34.2%, 44.7%, 46.3%, and 31.4%; febrile neutropenia: 15.8%, 28.9%, 29.3%, and 34.3%). Rates of ≥grade 3 infections were 21.1%, 26.3%, 36.6%, and 31.4% for Q1-Q4. In InO-treated pts, rates of ≥grade 3 hyperbilirubinemia were similar for the lower quartiles (Q1-Q3: 5.3%, 7.9%, and 2.4%; 11.4% for Q4); rates of ≥grade 3 veno-occlusive liver disease (VOD)/sinusoidal obstruction syndrome (SOS) within 2 years of randomization regardless of causality were 13.2%, 7.9%, 7.3%, and 17.1% in Q1-Q4, respectively; 3 grade 5 VOD/SOS events occurred in Q1, 2 in Q4, and 0 in Q2 and Q3.
Conclusions: In general, the results showed improvement in measures of efficacy for InO over SC that was comparable across all 4 CD22 positivity quartiles per local lab assessments. For DoR, PFS, and OS, there was a suggestion of greater benefit for pts in higher CD22 positivity quartiles treated with InO, though these analyses are limited by the small sample size. These trends are in alignment with those previously presented for central lab CD22 positivity and receptor density (Blood 2017;130[Suppl 1]:1272). Overall, InO demonstrated a favorable benefit/risk profile for pts with R/R B cell precursor ALL independent of local lab CD22 positivity.
Kantarjian:Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; Pfizer: Honoraria, Research Funding; Cyclacel: Research Funding; Ariad: Research Funding; Novartis: Research Funding; Takeda: Honoraria; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Agios: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharma: Research Funding; BMS: Research Funding; Immunogen: Research Funding. Stock:Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; UpToDate: Honoraria; Research to Practice: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Daiichi: Membership on an entity's Board of Directors or advisory committees. Cassaday:Seattle Genetics: Other: Spouse's disclosure: employment, stock and other ownership interests. DeAngelo:Celgene: Consultancy; Jazz Pharmaceuticals Inc: Consultancy; Takeda Pharmaceuticals: Consultancy; Abbvie: Research Funding; Shire: Consultancy; Amgen: Consultancy; GlycoMimetics: Research Funding; Incyte: Consultancy; Novartis: Consultancy, Research Funding; Blueprint: Consultancy, Research Funding; Pfizer: Consultancy. Jabbour:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding. O'Brien:Sunesis: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Eisai: Consultancy; Acerta: Research Funding; TG Therapeutics: Consultancy, Research Funding; Alexion: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Aptose Biosciences, Inc: Consultancy; GlaxoSmithKline: Consultancy; Astellas: Consultancy; Kite: Research Funding; Janssen: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Amgen: Consultancy; Regeneron: Research Funding; Verastem: Consultancy; Vaniam Group LLC: Consultancy; Celgene: Consultancy. Stelljes:Novartis: Honoraria; MDS: Consultancy; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding. Wang:Pfizer: Employment, Equity Ownership. Paccagnella:Pfizer: Employment, Equity Ownership. Nguyen:Navigate BioPharma Services, Inc., a Novartis Subsidiary: Employment; Novartis: Equity Ownership. Sleight:Pfizer: Employment, Equity Ownership. Vandendries:Pfizer: Employment, Equity Ownership. Neuhof:Pfizer: Employment, Equity Ownership. Laird:Pfizer: Employment, Equity Ownership. Advani:Amgen: Research Funding; Abbvie: Research Funding; Macrogenics: Research Funding; Pfizer: Honoraria, Research Funding; Glycomimetics: Consultancy, Research Funding; Kite Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal