Background: Bruton tyrosine kinase (BTK) is a key component of the B-cell receptor signaling pathway. The BTK inhibitors ibrutinib and acalabrutinib have been approved for the treatment of various B-cell malignancies. Zanubrutinib is a potent and highly specific next-generation BTK inhibitor currently in Phase 3 clinical testing. Achieving complete and sustained target engagement with respect to BTK receptor occupancy is thought to be an important factor for attaining deeper and durable clinical responses with the BTK inhibitors. Testing for BTK occupancy in peripheral blood mononuclear cells (PBMC) can be assessed in clinical studies. However, it is difficult to sample deep target tissues such as bone marrow (BM) and lymph nodes (LN) in patients to assess receptor occupancy. The objectives of this work were (1) to develop a QSP model to describe and predict the BTK receptor occupancy in PBMC, BM and LN after dosing with these BTK inhibitors in patients with B-cell malignancies, (2) to understand the impact of different dosing regimens (once daily (QD) v/s twice daily (BID)) on BTK receptor occupancy.
Methods: A mathematical model was developed that describes: (1) pharmacokinetics (PK) of BTK inhibitors; (2) intracellular concentration of BTK inhibitors in PBMC, BM and LN; (3) binding of BTK inhibitors with BTK including competition with ATP and (4) BTK degradation/turnover rate. This QSP model includes ibrutinib with its metabolite PCI-45227, acalabrutinib with its metabolite ACP-5862, and zanubrutinib. Previously developed minimal Physiologically-based Pharmacokinetic (PBPK) models were used to reproduce PK of BTK inhibitors. Intracellular concentrations of BTK inhibitors were described in the model in accordance with PBPK equations for small molecules (Rodgers and Rowland. J Pharm Sci. 2006) and on the basis of their physico-chemical properties. Parameters and interindividual variability in parameter values were taken from previously developed models and estimated using experimental data. Sensitivity analyses using key model parameters such as BTK degradation/turnover rate were performed. The model was validated using available in vitro and clinical data on BTK occupancy.
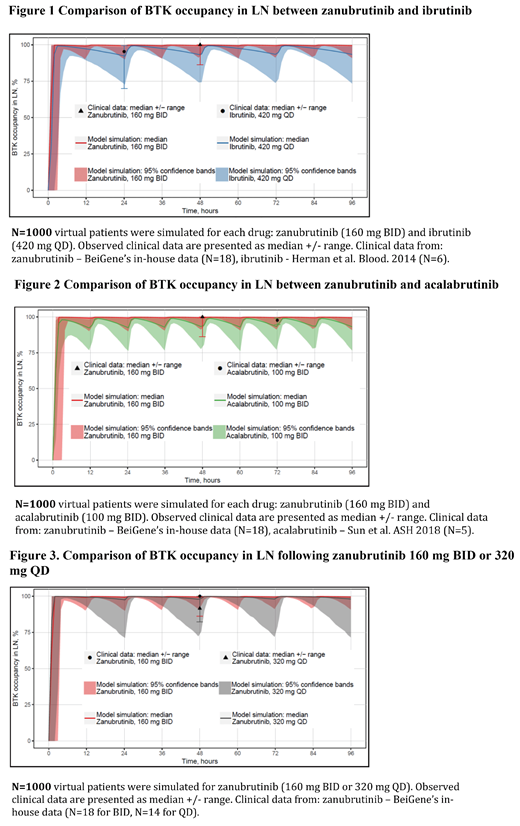
Results: The QSP model accurately described the observed clinical data on PK of BTK inhibitors during treatment with different doses and regimens of ibrutinib, acalabrutinib and zanubrutinib. There was good agreement between model prediction and observed BTK occupancy in PBMC and LN (Figure 1 and Figure 2). Zanubrutinib (160 mg BID) was predicted to result in higher median BTK occupancy with less variability in both PBMC and LN than ibrutinib (420 mg QD, 560 mg QD) and acalabrutinib (100 mg BID). In the absence of clinical BTK occupancy data in BM, the model predicted that steady-state trough BTK occupancy in BM were also predicted to be higher after zanubrutinib 160 mg BID administration, than those after ibrutinib 560 mg or 420 mg QD administration, and after acalabrutinib 100 mg BID administration. These differences are attributed to the PK properties (higher AUC and fraction unbound in plasma), binding properties to BTK (Kd, inactivation rate constant), and higher lipophilicity for zanubrutinib. The model predicted that 160 mg BID of zanubrutinib resulted in higher median trough BTK occupancy than 320 mg QD with less variability which is consistent with the observed clinical data (Figure 3).
Conclusions: A QSP model was successfully developed and validated, which predicted higher median BTK occupancy with less variability for zanubrutinib in PBMC, BM and LN than ibrutinib and acalabrutinib. A BID dosing regimen produces higher BTK occupancy than a QD regimen over the dosing interval. Ongoing clinical trials with 160 mg BID zanubrutinib will help to determine if improved BTK occupancy in these peripheral and deep target tissues translate into improvements in clinical outcomes.
Sahasranaman:BeiGene: Employment, Equity Ownership. Demin:BeiGene USA, Inc.: Consultancy; InSysBio LLC: Employment. Shchelokov:InSysBio LLC: Employment; BeiGene USA, Inc.: Consultancy. Musatova:BeiGene USA, Inc.: Consultancy; InSysBio LLC: Employment. Budha:BeiGene Ltd.: Employment, Equity Ownership. Ou:BeiGene: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal