Introduction:
Ibrutinib, an oral, selective inhibitor of Bruton's tyrosine kinase (BTK), dramatically improved
Progression-free survival (PFS) and Overall survival (OS) compared with immunochemotherapy in CLL both in first line and relapsed/refractory patients. However, some patients did progress on ibrutinb with dismal outcome. The underlying mechanism remains to be investigated beyond evolving of BTK and/or PLCg2 mutation, the dysfunction of apoptotic protein and mitochondrial apoptotic dependencies may be involves in ibrutinib resistance. PTBP1 (Polypyrimidine tract binding protein 1), a splicing factor, was found to be necessary for B cell selection in germinal centers. Knocking out PTBP1 in B cell resulted in impaired BCR-mediated B-cell activation and antibody production. Here, we investigate the regulation of PTBP1 on alternative splicing of apoptotic protein and its implications in CLL and ibrutinib resistance.
Methods:
Eighty-one CLL patients and 5 healthy controls were enrolled in this study from January 2010 to May 2018. The PTBP1 mRNA expression was measured by real-time polymerase chain reaction (RT-PCR) and Western-blot. We analyzed the PTBP1 expression with established CLL prognostic factors such as p53 and IGHV mutation status, and treatment to first treatment (TTFT). Resistant MEC-1 cell line was established by intermittently incubating with ibrutinib at a low concentration for short intervals and then gradually increased to 2-fold of IC50 value. Cells were allowed to recover every time after washing off the drug. RT-PCR was performed for both long and short isoform of MCL-1 by using specific primer in both parent and resistant cell lines and series ibrutinb-treated (both sensitive and resistant) patients' primary cells. Resistant MEC-1 cell line was cultured in RPMI 1640 without ibrutinib for 48hrs before transfection, siRNA targeting with PTBP1 mRNA and non-targeting siRNA were transfected into cells by using lipofectamine 3000. The transfection efficiency were verified by Western blot after 24 h and ibrutinib was added into resistant cell line. Apoptosis was then analyzed using flow cytometry (FCM) after 24 hrs. Receiver operating characteristic curve (ROC) and area under the ROC curve (AUC) were established to verify the best cut-off value in differentiating the high or low expression of PTBP1 mRNA. Time-to-first-treatment (TTFT) interval was defined as interval from diagnosis to first treatment. All statistical analyses were performed using the SPSS software program.
Results:
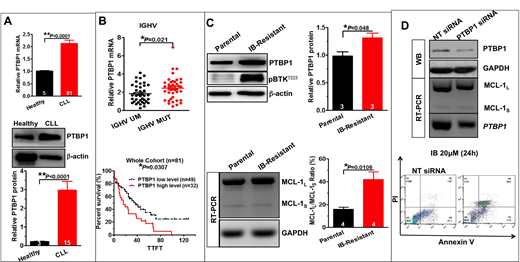
The expression of PTBP1 in CLL primary patients was significantly increased than 5 healthy donors (p < 0.01)(A). Patients with IGHV-mutated had higher level of PTBP1 as compared with patients with IGHV-unmutated (p < 0.05). Furthermore, Higher level of PTBP1 was associated with shorter TTFT in whole cohort, also in IGHV-mutated and unmutated subgroup (p < 0.05)(B). We further demonstrated that PTBP1 was aberrant expressed in ibrutinib resistant MEC-1 cell line or ibrutinib resistant primary patients' samples, as compared with parent cell line or patients' baseline samples. We also found the dysregulation of alternative splicing of MCL-1 in ibrutinib resistant models, presented with increased anti-apoptotic MCL-1L and decreased pro-apoptotic MCL-1S(C). Moreover, knocking down of PTBP1 sensitized CLL to ibrutinib via switching alternative splicing of MCL-1 to its pro-apoptotic isform MCL-1S(D).
Conclusions:
The splicing factor PTBP1 is involved in the pathogenesis of CLL. Its aberrant expression may lead to the dysregulation of alternative splicing of MCL-1, resulted in increased MCL-1L/s ratio.
PTBP1 can be as a promising target for the treatment of CLL patients progressed on ibrutinib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal