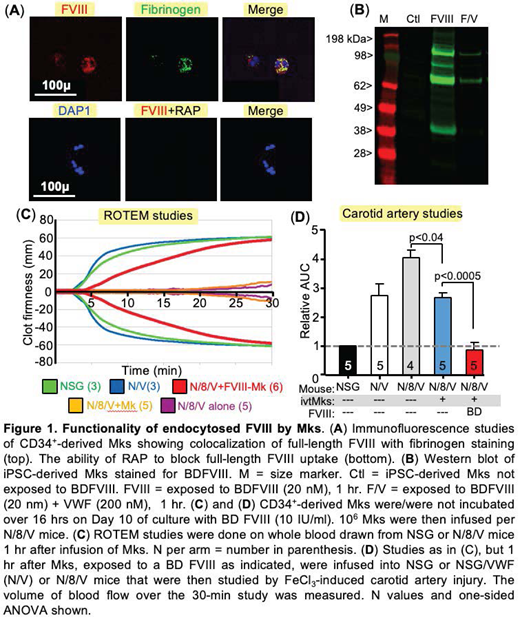
Human platelets endocytose coagulation factor (F) V into their α-granules, in part, via low-density lipoprotein (LDL) receptor-related protein 1 (LRP1). In contrast to humans, mouse FV is endogenously expressed during megakaryopoiesis and stored in α-granules. The FV-related coagulation factor FVIII is not endogenously expressed in megakaryocytes (Mks) and thought not to be endocytosed by human or murine Mks/platelets. The lack of FVIII endocytosis is surprising as LRP1 on multiple cell types is involved in FVIII uptake and clearance. We and others have shown that FVIII can be ectopically expressed during human or murine megakaryopoiesis and stored in α-granules. These platelets are effective in the delivery of FVIII to sites of vascular injury and improve outcome in hemophilia A (HA) mice even in the presence of circulating inhibitors. Thus, it has been proposed that lentiviral bone marrow gene therapy to ectopically express FVIII during megakaryopoiesis may be a curative strategy especially for patients with HA and intractable inhibitors. However, we have shown that pFVIII has a toxic effect on Mks during intracellular processing in the endoplasmic reticulum/Golgi, limiting platelet yield and pFVIII levels. We have recently shown that exogenous FV can be taken up by in vitro differentiated CD34+- and by induced pluripotent stem cell (iPSC)-derived Mks and asked if the same can be done with FVIII. We found that Mks can take up full-length FVIII (Advate) (Fig. 1A), B-domainless (BD) FVIII (Xyntha, not shown) and BD FVIIIR1645H (not shown), a FVIII mutant we have shown is particularly effective when released by platelets. Uptake is half maximum at 0.25 IU/ml following overnight incubation. Endocytosed FVIII colocalizes with labeled fibrinogen uptake (Fig. 1A), supporting its localization to α-granules, and this FVIII uptake can be blocked by receptor-associated protein (RAP), a blocker of LDL receptor family members, including LRP1 (Fig. 1A) or by including FVIII's carrier protein von Willebrand factor (VWF) (Fig. 1B). To test the biological efficacy of endocytosed pFVIII, we took advantage of our previous studies showing that infused Mks into mice release highly functional platelets after becoming entrapped in the lungs. To focus our studies on the released human platelets and the hemostatic efficacy of the endocytosed pFVIII, we used immunodeficient NOD-scid IL2rgnull (NSG) mice that were also FVIII-deficient and that only expressed mutant VWF that binds human, but not mouse, platelet glycoprotein Ib/IX (VWFR1326H) (N/8/V mice). We infused 1x106 human FVIII-endocytosed Mks into these N/8/V mice resulting in ~1-10% being human platelets in recipient mice. We studied hemostatic efficacy by rotational thromboelastography (ROTEM) and demonstrated that the in vivo-released FVIII from released human platelets within the N/8/V blood corrected hemostasis in this system (Fig. 1C). These FVIII-containing platelets fully corrected clotting as well in a FeCl3 carotid artery injury model (Fig. 1D). In summary, we found that in vitro-grown human Mks can endocytose FVIII from the media into their α-granules in sufficient amounts to have potential clinical application in the care of HA patients. This endocytosis is likely via LRP1. FVIII is not found normally in platelets likely because of a combination of the following: 1) in plasma, FVIII it is bound to VWF, 2) circulating platelets also lack LRP1, and 3) in the marrow, there is little or no free FVIII for endocytosis by LRP1-positive Mks. We propose that endocytosed FVIII by Mks can be an important clinical application of commercial, in vitro-grown Mks for patients with HA and inhibitors who need supplemental long-lasting, hemostatic support to their emicizumab without the thrombotic risks of FVIII-bypassing agents.
Sabatino:Spark Therapeutics: Patents & Royalties. Camire:Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal